GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: tunicamycin A | tunicamycin B2 | tunicamycin V
Compound class:
Natural product
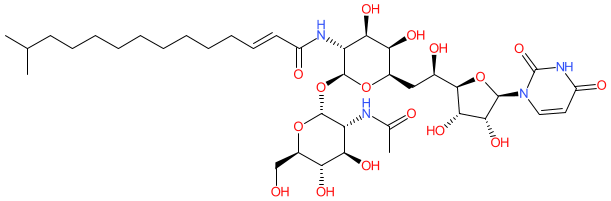
Comment: Tunicamycin is a nucleoside antibacterial compound, originally isolated from culture broth of Streptomyces lysosuperificus [1]. It inhibits phospho-N-acetylmuramoyl-pentapeptide-transferase (MraY, translocase I), an essential enzyme in bacterial peptidoglycan biosynthesis.
|
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Natural product |
Synonyms  |
| tunicamycin A | tunicamycin B2 | tunicamycin V |
Database Links  |
|
| Specialist databases | |
Antibiotic DB

|
Tunicamycin |
| Other databases | |
| BindingDB Ligand | 50537412 |
| CAS Registry No. | 66054-36-2 (source: Scifinder) |
| ChEBI | CHEBI:64250 |
| ChEMBL Ligand | CHEMBL4534172 |
| GtoPdb PubChem SID | 500839920 |
| PubChem CID | 11104835 |
| RCSB PDB Ligand | 9LH |
| Search Google for chemical match using the InChIKey | MEYZYGMYMLNUHJ-DIRMKAHISA-N |
| Search Google for chemicals with the same backbone | MEYZYGMYMLNUHJ |
| UniChem Compound Search for chemical match using the InChIKey | MEYZYGMYMLNUHJ-DIRMKAHISA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | MEYZYGMYMLNUHJ-DIRMKAHISA-N |
| Wikipedia | Tunicamycin |
Product suppliers
View disclaimer
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖