GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Neuropeptide FF/neuropeptide AF receptors: Introduction
General
The Neuropeptide FF receptor family is a member of the G protein-coupled receptor superfamily containing two subtypes, NPFFR1 and NPFFR2, which exhibit high affinities for Neuropeptide FF (NPFF) and Rfamide related peptides (RFRP). NPFFR1 is broadly distributed in the central nervous system with the highest levels found in the limbic system and the hypothalamus. NPFFR2 is present in high density, particularly in mammals in the superficial layers of the spinal cord where it is involved in nociception and modulation of opioid functions [3,25,30,43].
NPFF peptides
Neuropeptide FF (NPFF, FLFQPQRFamide) is representative of a family of RFamide peptides that regulate pain, cardiovascular functions, appetite, thirst, and body temperature. NPFF is an amidated neuropeptide first isolated from bovine brain [57]. Genes encoding two precursors, NPFFA [37,55] and NPFFB [14,25] have been cloned in mammals. NPFFB products are also referred as RFamide-related peptides (RFRP) or gonadotropin-inhibitory hormone (GnIH) and regulate pain modulation, stress response and hypothalamic-pituitary-gonadal axis [22,25,53]; see [50] for review]. See table 1 for peptides originating from these precursors in humans.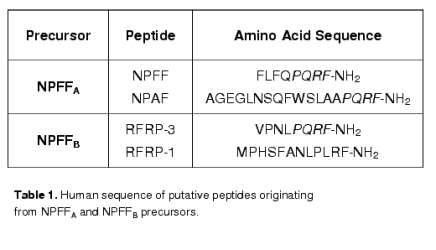
The NPFFA precursor could maturate peptides with a C-terminal PQRF-NH2 sequence and an N-terminal sequence that differs in different species. Several peptides contained in the NPFFA precursor have been biochemically identified in various species (see table 2 and [58] for review).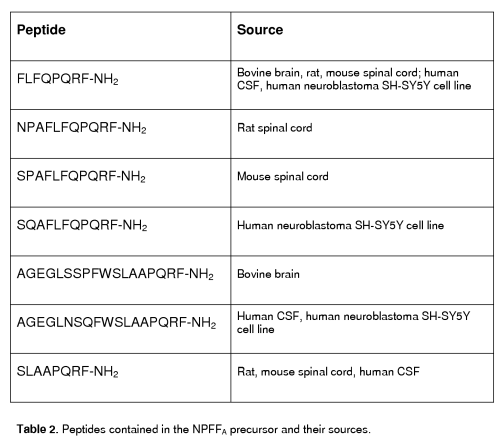
The NPFFB precursor is a precursor for RFRP or GnIH [14,50]. It matures peptides with a C-terminal LPXRF-NH2 (X= L or Q) sequence. Several peptides contained in the NPFFB precursor have been biochemically identified in various mammalian species (see Table 3 and [50] for review).
| Name | Peptide | Source |
| Human RFRP-1 | MPHSFANLPLRF-NH2 | Human brain [53] |
| Human RFRP-3 | VPNLPQRF-NH2 | Human brain [53] |
| Macaque RFRP-3 | SGRNMEVSLVRQVLNLPQRF-NH2 | Macaque brain [52] |
| Bovine RFRP-1 | SLTFEEVKDWAPKIKMNKPVVNKMPPSAANLPLRF-NH2 | Bovine brain [9] |
| Bovine RFRP-3 | AMAHLPLRLGKNREDSLSRWVPNLPQRF-NH2 | Bovine brain [59] |
| Rat RFRP-3 | ANMEAGTMSHFPSLPQRF-NH2 | Rat brain [54] |
| Hamster RFRP-1 | SPAPANKVPHSAANLPLRF-NH2 | Hamster brain [51] |
| Hamster RFRP-3 | TLSRVPSLPQRF-NH2 | Hamster brain [51] |
Receptor family
Two NPFF receptors have been identified; NPPF2 (also referred to as HLWAR77, NPGPR and GPR74) [3,8] and NPFF1 (also known as OT7TO22) [3,14].
Receptor structure
NPFF1 and NPFF2 receptors belong to the G protein-coupled receptor family. They are about 50% identical and are most similar to neuropeptide Y and orexin receptors (30-35% homology). Variants of the NPFF2 receptor in N- and/or C- terminals have been reported [60]. Comparative studies of the pharmacological profiles of both receptors indicate that NPFF1 receptors are not very discriminative towards the endogenous peptides from both precursors NPFFA and NPFFB. However, peptides from the NPFFA precursor display a higher affinity for the NPFF2 receptor and, conversely, peptides from the NPFFB precursor slightly prefer the NPFF1 receptor [25,28]. NPFF receptor activity in recombinant cells, especially NPFF1 activity, is reversed by BIBP3226, a selective antagonist for neuropeptide Y, Y1 receptors [27-28]. In vitro and in vivo pharmacological activities of NPFF receptors are reversed by the potent antagonist RF9 [16,45].
Selective agonists with high affinity for each NPFF receptor are available as well as selective radioiodinated ligands [28]. Moreover, the frog pancreatic polypeptide exhibits nanomolar affinity for NPFF2 receptors.
Signal transduction
NPFF receptors are coupled to G proteins and regulate adenylyl cyclase in recombinant cell lines (CHO, HEK 293, SH-SY5Y) [3,25,28-29]. NPFF receptors are also coupled to voltage-gated N-type Ca2+ channels [20]. At this time, the endogenous intracellular signalling pathways activated by NPFF receptors in neurones are still unknown.
Much data indicate that, at the cellular level, stimulation of NPFF receptors inactivates opioid receptors by a molecular mechanism which is still not understood. In acutely dissociated neurons, NPFF2 receptors specifically counteract N-type Ca2+ channel inhibition by opioids [40,42]. In SH-SY5Y neuroblastoma cells stably expressing human NPFF receptors, NPFF agonists also reduce the inhibitory effect of μ and δ-opioid receptor activation on an N-type Ca2+ channel [20,29]. These regulations could be due in part to receptor heteromerisation since NPFF2 receptors have been shown to physically interact with mu-opioid receptors [41] and induce their trans-phosphorylations [32].
The NPFF analog 1DMe blocks presynaptic δ-opioid autoreceptors in the spinal cord, leading to an increase in K+-evoked met-enkephalin release [2], that could explain the analgesic effect of an intrathecal injection of 1DMe [13]. 1DMe also inhibits the reduction induced by morphine, but not by noradrenaline, of the electrically evoked acetylcholine release in the guinea pig myenteric plexus [49]. Furthermore, on isolated neurons from rat periventricular and dorsal raphe nuclei that co-express nociceptin receptors and NPFF1 or NPFF2 receptors, respectively, NPFF analogs inhibit the nociceptin-induced reduction of N-type Ca2+ channel conductance [40,42].
NPFF causes an increase in miniature inhibitory postsynaptic currents in magnocellular neurosecretory cells of the paraventricular nucleus indicating a presynaptic locus of action [15].
NPFF1 couples with Gαi to inhibit adenylyl cyclase (AC) [14], and reduces the activities of cAMP-dependent protein kinase (PKA) and mitogen-activated protein kinase (MAPK) signaling cascade. The inhibitory action of RFRPs on gonadotropin gene expression was suggested to be mediated by an inhibition of AC/cAMP/PKA-dependent extracellular signal-regulated kinase (ERK) pathway [47]. In the sheep, RFRP-3 can inhibit both gonadotropin-releasing hormone (GnRH)-induced intracellular Ca2+ increase and ERK phosphorylation, impacting GnRH-induced gonadotropin release and synthesis [44].
Tissue distribution
NPFF receptors [10,12] and mRNA [3] are localised in brain regions known to contain opioid receptors and to be involved in nociception control. A comparative autoradiographic study [12], using two radioligands, [125I]YVP and [125I]EYF selective for NPFF1 and NPFF2 receptors, respectively, has revealed that NPFF2 receptors are mainly expressed in the rat central nervous system (especially in the dorsal horn of the spinal cord, the parafascicular and laterodorsal nuclei of the thalamus and some hypothalamus nuclei), indicating that most of the pharmacological effects of NPFF in this species are due to the NPFF2 receptor.
In situ hybridization, autoradiography, and immunohistochemistry in the rat brain have shown that NPFF1 is expressed in various brain regions including the limbic system of telencephalon, diencephalon, mesencephalon as well as the pituitary [3,25].
Comparison of NPFF1 and NPFF2 receptor distribution in rat, mouse, octodon, rabbit, guinea-pig and monkey reveals important species differences [11] and shows that the pattern of NPFF2 receptors is consistent with a potential role of NPFF in the modulation of sensory inputs while NPFF1 could participate in neuroendocrine functions.
Functions
NPFF is involved in several physiological functions such as hormonal modulation, cardiovascular and thermal regulation and food intake [30]. Furthermore, many pharmacological data suggest that NPFF plays an important role in pain modulation and acts mainly through the regulation of the opioid system [33,36,43]. A close relationship between NPFF and opioid systems exists in the central nervous system, since NPFF analogs increase or decrease opioid effects, depending on dose and site of administration [43]. In general, intracerebroventricular injected analogues exert pronociceptive activity while intrathecal injection induces analgesia [58]. The NPFF system is therefore considered to be an opioid-modulating system involved in homeostasis that counteracts the action of opioids and thus plays a role in opiate tolerance [36,43]. Furthermore, NPFF injected during conditioned place preference, influences the development of rewarding effects of morphine [26]. The NPFF antagonist RF9 prevents the development of analgesia tolerance and decreases the withdrawal syndrome following chronic morphine treatment [7]. The stimulation of NPFF receptors in the ventral tegmental area blocks morphine-induced release of dopamine in the rat [21] while i.c.v injections of an NPFF analog inhibits morphine-induced expression of c-fos in the same brain region in mice [31].
NPFF also displays potent effects in the periphery. Intravenous injection of NPFF causes a transient rise in arterial blood pressure and heart rate in rats [1,39].
The ICV administration of NPFF reduces food intake and the combination of NPFF and naloxone shows no additivity in the anorexigenic effect, suggesting that NPFF and naloxone reduced food intake by a common mechanism. This suggests that NPFF may function as an endogenous anorexigenic peptide with antiopioid function [23,35,48]. One of the emerging themes from recent research is that NPFF endogenous peptides are involved in control of feeding behaviour both in invertebrates and in vertebrates [5].
Abundant RFRP-immunoreactive (ir) neuronal fibers are observed in the median eminence (ME) of sheep [4], macaque [52], and humans [53]. RFRP-3, a mammalian GnIH ortholog, inhibits gonadotropin synthesis and/or release from cultured pituitaries in sheep [44] and cattle [18]. Peripheral administration of RFRP-3 also inhibits gonadotropin release in sheep [4] and cattle [18]. It was further shown that NPFF1 mRNA is expressed in gonadotrophs in the human pituitary [53]. Recent study has also shown that RFRP-3 is secreted in the portal blood in a pulsatile manner in sheep [46]. Taken together, it is likely that RFRP-3 directly acts on the pituitary to inhibit gonadotropin secretion from the pituitary at least in these mammalian species. On the contrary, it is thought that RFRP-3 does not act directly on the pituitary in rodents, because there are relatively few or no RFRP-ir fibers in the ME [24,51]. However, there are also several studies indicating that RFRP-3 can directly inhibit gonadotropin release from the pituitary [34] (see [50] for review).
Immunohistochemical studies using light and confocal microscopy indicate that neuronal axon terminals containing RFRP-3 are in probable contact with GnRH neurons in mammals [24,51-53]. NPFF1 also is expressed in GnRH neurons in hamsters [51]. Together with the observation that central administration of RFRP-3 inhibits the release of gonadotropin in hamsters [24] [51] and rats [17], RFRP-3 may inhibit the secretion of gonadotropins by decreasing GnRH neuronal activity in addition to regulating the gonadotroph directly. Direct application of RFRP-3, a mammalian GnIH ortholog, to GnRH cells in cultured mouse brain slices decreases firing rate in a subpopulation of cells, further indicating a direct action of RFRP-3 on GnRH neurons. In addition, RFRP-3 inhibits firing of kisspeptin-activated vGluT2 (vesicular glutamate transporter 2)-GnRH neurons as well as of kisspeptin-insensitive GnRH neurons in mice [6,56]. Central administration of RF9, an antagonist of NPFF receptors, to rats and mice led to marked increases in gonadotropin concentrations, providing a pronounced role of RFRP-3 as a key regulator of the reproductive axis in mammals [38].
Central administrations of RFRP-3 inhibit reproductive behavior of male rats [17]. On the other hand, central administrations of RFRP-3 stimulate feeding behavior in rats [17,34]. There is also a report that central administration of RFRP-3 can stimulate adrenocorticotropic hormone and oxytocin release in rats [19]. It was shown that RFRP-ir fibers project to various neurons in the brain, such as dopamine and/or proopiomelanocortin neurons in the rat, sheep and macaque [52], indicating multiple functions of GnIH and its orthologs in the brain of mammals.
References
1. Allard M, Labrouche S, Nosjean A, Laguzzi R. (1995) Mechanisms underlying the cardiovascular responses to peripheral administration of NPFF in the rat. J Pharmacol Exp Ther, 274 (1): 577-83. [PMID:7616447]
2. Ballet S, Mauborgne A, Gouardères C, Bourgoin AS, Zajac JM, Hamon M, Cesselin F. (1999) The neuropeptide FF analogue, 1DME, enhances in vivo met-enkephalin release from the rat spinal cord. Neuropharmacology, 38 (9): 1317-24. [PMID:10471085]
3. Bonini JA, Jones KA, Adham N, Forray C, Artymyshyn R, Durkin MM, Smith KE, Tamm JA, Boteju LW, Lakhlani PP et al.. (2000) Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J Biol Chem, 275 (50): 39324-31. [PMID:11024015]
4. Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K et al.. (2008) Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology, 149 (11): 5811-21. [PMID:18617613]
5. Dockray GJ. (2004) The expanding family of -RFamide peptides and their effects on feeding behaviour. Exp Physiol, 89 (3): 229-35. [PMID:15123557]
6. Ducret E, Anderson GM, Herbison AE. (2009) RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology, 150 (6): 2799-804. [PMID:19131572]
7. Elhabazi K, Trigo JM, Mollereau C, Moulédous L, Zajac JM, Bihel F, Schmitt M, Bourguignon JJ, Meziane H, Petit-demoulière B et al.. (2012) Involvement of neuropeptide FF receptors in neuroadaptive responses to acute and chronic opiate treatments. Br J Pharmacol, 165 (2): 424-35. [PMID:21718302]
8. Elshourbagy NA, Ames RS, Fitzgerald LR, Foley JJ, Chambers JK, Szekeres PG, Evans NA, Schmidt DB, Buckley PT, Dytko GM et al.. (2000) Receptor for the pain modulatory neuropeptides FF and AF is an orphan G protein-coupled receptor. J Biol Chem, 275 (34): 25965-71. [PMID:10851242]
9. Fukusumi S, Habata Y, Yoshida H, Iijima N, Kawamata Y, Hosoya M, Fujii R, Hinuma S, Kitada C, Shintani Y et al.. (2001) Characteristics and distribution of endogenous RFamide-related peptide-1. Biochim Biophys Acta, 1540 (3): 221-32. [PMID:11583817]
10. Gouardères C, Mollereau C, Tafani JA, Mazarguil H, Zajac JM. (2001) [(125)I]EYF: a new high affinity radioligand to neuropeptide FF receptors. Peptides, 22 (4): 623-9. [PMID:11311733]
11. Gouardères C, Puget A, Zajac JM. (2004) Detailed distribution of neuropeptide FF receptors (NPFF1 and NPFF2) in the rat, mouse, octodon, rabbit, guinea pig, and marmoset monkey brains: a comparative autoradiographic study. Synapse, 51 (4): 249-69. [PMID:14696013]
12. Gouardères C, Quelven I, Mollereau C, Mazarguil H, Rice SQ, Zajac JM. (2002) Quantitative autoradiographic distribution of NPFF1 neuropeptide FF receptor in the rat brain and comparison with NPFF2 receptor by using [125I]YVP and [(125I]EYF as selective radioligands. Neuroscience, 115 (2): 349-61. [PMID:12421602]
13. Gouardères C, Sutak M, Zajac JM, Jhamandas K. (1993) Antinociceptive effects of intrathecally administered F8Famide and FMRFamide in the rat. Eur J Pharmacol, 237 (1): 73-81. [PMID:8102975]
14. Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y et al.. (2000) New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol, 2 (10): 703-8. [PMID:11025660]
15. Jhamandas JH, MacTavish D, Harris KH. (2006) Neuropeptide FF (NPFF) control of magnocellular neurosecretory cells of the rat hypothalamic paraventricular nucleus (PVN). Peptides, 27 (5): 973-9. [PMID:16517015]
16. Jhamandas JH, Simonin F, Bourguignon JJ, Harris KH. (2007) Neuropeptide FF and neuropeptide VF inhibit GABAergic neurotransmission in parvocellular neurons of the rat hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol, 292 (5): R1872-80. [PMID:17289819]
17. Johnson MA, Tsutsui K, Fraley GS. (2007) Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav, 51 (1): 171-80. [PMID:17113584]
18. Kadokawa H, Shibata M, Tanaka Y, Kojima T, Matsumoto K, Oshima K, Yamamoto N. (2009) Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domest Anim Endocrinol, 36 (4): 219-24. [PMID:19328642]
19. Kaewwongse M, Takayanagi Y, Onaka T. (2011) Effects of RFamide-related peptide (RFRP)-1 and RFRP-3 on oxytocin release and anxiety-related behaviour in rats. J Neuroendocrinol, 23 (1): 20-7. [PMID:21029217]
20. Kersanté F, Mollereau C, Zajac JM, Roumy M. (2006) Anti-opioid activities of NPFF1 receptors in a SH-SY5Y model. Peptides, 27 (5): 980-9. [PMID:16488058]
21. Kersanté F, Wang JY, Chen JC, Mollereau C, Zajac JM. (2011) Anti-opioid effects of neuropeptide FF receptors in the ventral tegmental area. Neurosci Lett, 488 (3): 305-9. [PMID:21111027]
22. Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. (2009) Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci USA, 106 (27): 11324-9. [PMID:19541621]
23. Kotani M, Mollereau C, Detheux M, Le Poul E, Brézillon S, Vakili J, Mazarguil H, Vassart G, Zajac JM, Parmentier M. (2001) Functional characterization of a human receptor for neuropeptide FF and related peptides. Br J Pharmacol, 133 (1): 138-44. [PMID:11325803]
24. Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. (2006) Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA, 103 (7): 2410-5. [PMID:16467147]
25. Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, Jiang Q, Zeng Z, Jacobson M, Williams Jr DL, Yu H et al.. (2001) Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem, 276 (40): 36961-9. [PMID:11481330]
26. Marchand S, Betourne A, Marty V, Daumas S, Halley H, Lassalle JM, Zajac JM, Frances B. (2006) A neuropeptide FF agonist blocks the acquisition of conditioned place preference to morphine in C57Bl/6J mice. Peptides, 27 (5): 964-72. [PMID:16494968]
27. Mollereau C, Gouardères C, Dumont Y, Kotani M, Detheux M, Doods H, Parmentier M, Quirion R, Zajac JM. (2001) Agonist and antagonist activities on human NPFF(2) receptors of the NPY ligands GR231118 and BIBP3226. Br J Pharmacol, 133 (1): 1-4. [PMID:11325787]
28. Mollereau C, Mazarguil H, Marcus D, Quelven I, Kotani M, Lannoy V, Dumont Y, Quirion R, Detheux M, Parmentier M et al.. (2002) Pharmacological characterization of human NPFF(1) and NPFF(2) receptors expressed in CHO cells by using NPY Y(1) receptor antagonists. Eur J Pharmacol, 451 (3): 245-56. [PMID:12242085]
29. Mollereau C, Mazarguil H, Zajac JM, Roumy M. (2005) Neuropeptide FF (NPFF) analogs functionally antagonize opioid activities in NPFF2 receptor-transfected SH-SY5Y neuroblastoma cells. Mol Pharmacol, 67 (3): 965-75. [PMID:15608144]
30. Mollereau C, Roumy M, Zajac JM. (2005) Opioid-modulating peptides: mechanisms of action. Curr Top Med Chem, 5: 341-355. [PMID:15857316]
31. Moulédous L, Frances B, Zajac JM. (2010) Modulation of basal and morphine-induced neuronal activity by a NPFF(2) selective agonist measured by c-Fos mapping of the mouse brain. Synapse, 64 (9): 672-81. [PMID:20336629]
32. Moulédous L, Froment C, Dauvillier S, Burlet-Schiltz O, Zajac JM, Mollereau C. (2012) GRK2 protein-mediated transphosphorylation contributes to loss of function of μ-opioid receptors induced by neuropeptide FF (NPFF2) receptors. J Biol Chem, 287 (16): 12736-49. [PMID:22375000]
33. Moulédous L, Mollereau C, Zajac JM. (2010) Opioid-modulating properties of the neuropeptide FF system. Biofactors, 36 (6): 423-9. [PMID:20803521]
34. Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. (2008) Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol, 199 (1): 105-12. [PMID:18653621]
35. Murase T, Arima H, Kondo K, Oiso Y. (1996) Neuropeptide FF reduces food intake in rats. Peptides, 17 (2): 353-4. [PMID:8801545]
36. Panula P, Kalso E, Nieminen M, Kontinen VK, Brandt A, Pertovaara A. (1999) Neuropeptide FF and modulation of pain. Brain Res, 848 (1-2): 191-6. [PMID:10612711]
37. Perry SJ, Yi-Kung Huang E, Cronk D, Bagust J, Sharma R, Walker RJ, Wilson S, Burke JF. (1997) A human gene encoding morphine modulating peptides related to NPFF and FMRFamide. FEBS Lett, 409 (3): 426-30. [PMID:9224703]
38. Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, Dijcks FA, Blomenröhr M, Pinilla L, van Noort PI et al.. (2010) Characterization of the potent gonadotropin-releasing activity of RF9, a selective antagonist of RF-amide-related peptides and neuropeptide FF receptors: physiological and pharmacological implications. Endocrinology, 151 (4): 1902-13. [PMID:20160130]
39. Roth BL, Disimone J, Majane EA, Yang HY. (1987) Elevation of arterial pressure in rats by two new vertebrate peptides FLFQPQRF-NH2 and AGEGLSSPFWSLAAPQRF-NH2 which are immunoreactive to FMRF-NH2 antiserum. Neuropeptides, 10 (1): 37-42. [PMID:3670567]
40. Roumy M, Garnier M, Zajac JM. (2003) Neuropeptide FF receptors 1 and 2 exert an anti-opioid activity in acutely dissociated rat dorsal raphe and periventricular hypothalamic neurones. Neurosci Lett, 348 (3): 159-62. [PMID:12932818]
41. Roumy M, Lorenzo C, Mazères S, Bouchet S, Zajac JM, Mollereau C. (2007) Physical association between neuropeptide FF and micro-opioid receptors as a possible molecular basis for anti-opioid activity. J Biol Chem, 282 (11): 8332-42. [PMID:17224450]
42. Roumy M, Zajac J. (1999) Neuropeptide FF selectively attenuates the effects of nociceptin on acutely dissociated neurons of the rat dorsal raphe nucleus. Brain Res, 845 (2): 208-14. [PMID:10536200]
43. Roumy M, Zajac JM. (1998) Neuropeptide FF, pain and analgesia. Eur J Pharmacol, 345 (1): 1-11. [PMID:9593588]
44. Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. (2009) Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology, 150 (12): 5549-56. [PMID:19808777]
45. Simonin F, Schmitt M, Laulin JP, Laboureyras E, Jhamandas JH, MacTavish D, Matifas A, Mollereau C, Laurent P, Parmentier M et al.. (2006) RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc Natl Acad Sci USA, 103 (2): 466-71. [PMID:16407169]
46. Smith JT, Young IR, Veldhuis JD, Clarke IJ. (2012) Gonadotropin-inhibitory hormone (GnIH) secretion into the ovine hypophyseal portal system. Endocrinology, 153 (7): 3368-75. [PMID:22549225]
47. Son YL, Ubuka T, Millar RP, Kanasaki H, Tsutsui K. (2012) Gonadotropin-inhibitory hormone inhibits GnRH-induced gonadotropin subunit gene transcriptions by inhibiting AC/cAMP/PKA-dependent ERK pathway in LβT2 cells. Endocrinology, 153 (5): 2332-43. [PMID:22374973]
48. Sunter D, Hewson AK, Lynam S, Dickson SL. (2001) Intracerebroventricular injection of neuropeptide FF, an opioid modulating neuropeptide, acutely reduces food intake and stimulates water intake in the rat. Neurosci Lett, 313 (3): 145-8. [PMID:11682148]
49. Takeuchi T, Fujita A, Roumy M, Zajac JM, Hata F. (2001) Effect of 1DMe, a neuropeptide FF analog, on acetylcholine release from myenteric plexus of guinea pig ileum. Jpn J Pharmacol, 86 (4): 417-22. [PMID:11569615]
50. Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. (2010) Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol, 31 (3): 284-95. [PMID:20211640]
51. Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. (2012) Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology, 153 (1): 373-85. [PMID:22045661]
52. Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. (2009) Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol, 517 (6): 841-55. [PMID:19844991]
53. Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. (2009) Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS ONE, 4 (12): e8400. [PMID:20027225]
54. Ukena K, Iwakoshi E, Minakata H, Tsutsui K. (2002) A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett, 512 (1-3): 255-8. [PMID:11852091]
55. Vilim FS, Aarnisalo AA, Nieminen ML, Lintunen M, Karlstedt K, Kontinen VK, Kalso E, States B, Panula P, Ziff E. (1999) Gene for pain modulatory neuropeptide NPFF: induction in spinal cord by noxious stimuli. Mol Pharmacol, 55 (5): 804-11. [PMID:10220558]
56. Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. (2009) Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol (Lond.), 587 (Pt 7): 1401-11. [PMID:19204051]
57. Yang HY, Fratta W, Majane EA, Costa E. (1985) Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc Natl Acad Sci USA, 82 (22): 7757-61. [PMID:3865193]
58. Yang HY, Iadarola MJ. (2006) Modulatory roles of the NPFF system in pain mechanisms at the spinal level. Peptides, 27 (5): 943-52. [PMID:16443306]
59. Yoshida H, Habata Y, Hosoya M, Kawamata Y, Kitada C, Hinuma S. (2003) Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim Biophys Acta, 1593 (2-3): 151-7. [PMID:12581859]
60. Zajac JM. (2001) Neuropeptide FF: new molecular insights. Trends Pharmacol Sci, 22 (2): 63. [PMID:11421201]






