GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Corticotropin-releasing factor receptors: Introduction
Introduction
The 41-amino peptide corticotropin-releasing factor (CRF) was first isolated from ovine hypothalamus [49]. Initially, the action of CRF appeared to be restricted to regulating ACTH secretion by the pituitary [17-18,43,49]. The discovery that CRF-expressing neurons existed in pathways outside of the hypothalamus, however, led to the realization that CRF's function extended far beyond the classical action of a hormone in the hypothalamic-pituitary adrenocortical axis [2,12,15,20,46,50,53]. It then became apparent that brain CRF neurotransmission was critical for behavioral, neuroendocrine, and autonomic responses to stress. In fact, CRF-induced activation of brain CRF1 receptors is both necessary and sufficient to define the stress response.
Urocortin 1, a second mammalian CRF-like peptide was identified following the sequencing of two novel CRF-like peptides urotensin I [25] and sauvagine [31] in fish and amphibians [34,45]. Subsequently, human, sheep, rat, and mouse urocortin 1 were shown to have 40-amino acid sequences [4,16,51,55] with high degrees of homology with fish urotensin I. While urocortin 1 is discretely expressed in the central nervous system this CRF-like peptide is widely distributed in the periphery [3,22].
In 2001, two novel urocortin-like peptides, urocortin 2 and urocortin 3 [26,42], were cloned from human and mouse cDNA libraries. At the same time, another group identified two similar peptides, which they named stresscopin (which is homologous with urocortin 3) and stresscopin-related peptide (which is homologous with urocortin 2) [21](see Table 1). The remarkably discrete expression of urocortin 2 (stresscopin-related peptide) and urocortin 3 (stresscopin) in the central nervous system does not conform with the broad distribution of CRF pathways and the discrete pattern of urocortin 1 pathways [21,26,42]. Furthermore, the central distribution of urocortins 1-3 and CRF2 receptor-expressing neurons suggests that all three peptides may serve as major CRF2 receptor ligands in highly localized and specific manner. High expression of urocortin 3 mRNA in the lateral septum and urocortin 2 mRNA in the locus coeruleus is particularly intriguing with regard to the possibility that CRF2 receptor-mediated mechanisms may modulate and oppose in certain situations neural stress responses evoked by CRF1 receptor activation. In the periphery, urocortin 2 mRNA is detected in the heart, adrenal gland, and peripheral blood cells [21,42]. The highest peripheral levels of urocortin 3 mRNA expression have been detected in the colon, pancreatic islet cells, muscle, adrenal gland, and skin [21,26]. Because urocortin 2 and urocortin 3 were discovered by molecular cloning strategies [21,26,42], their exact size has not been established yet. The structure of both precursor genes appears to predict 38-amino acid mature peptides [26,42] although one group [21] postulated the existence of N-terminally extended peptides 40 (urocortin 3) and 43 (urocortin 2) amino acids in length (Table 1). The final forms of post-translationally modified urocortin 2 and urocortin 3 peptides in brain and peripheral tissues remains to be determined. However, because the human and mouse homologues of both precursors are less conserved in the extended N-terminus than in the remaining sequence (Table 1), it seems more likely that urocortin 2 and urocortin 3 exist as 38 amino acid peptides. Like CRF
The CRF1 receptor
The CRF1 receptor, a 415-446 amino acid polypeptide, has been cloned from a variety of species ranging from fish to man [1,6-8,32,35,37-38,40,52,54]. The CRF1 receptor is widely expressed in the central nervous system with high levels of expression in cortex, cerebellum, hippocampus, basolateral amygdala, bed nucleus of thes stria terminalis, olfactory bulb and anterior pituitary [5,37,41]. In the periphery, CRF1 receptor mRNA is expressed at low levels in the skin, ovary, testis and adrenal gland [33,37,52].
Binding and functional studies using cell lines recombinantly or endogenously expressing CRF1 receptors revealed a distinct ligand-selective profile whereby human and ovine CRF, urocortin 1, urotensin I, and sauvagine all bind with high affinity to the mammalian CRF1 receptor and activate the cyclic AMP signaling pathway [8,13,16,39]. In contrast, urocortin 2 and urocortin 3 do not bind to or activate CRF1 receptors [9,21,26,42]. Therefore, CRF and urocortin 1 can be classified as the endogenous ligands for mammalian CRF1 receptors.
The CRF2 receptor
Complementary DNAs for the CRF2 receptor have also been isolated from large number of vertebrate species [1,8,23-24,26,28,30,36,40,44,48]. Three functional splice variants [9,28,48] have been identified for the mammalian CRF2 receptor. The CRF(2a) receptor variant is only expressed in non-mammalian species [1,8,36,40], while the 430-438 amino-acid CRF(2b) receptor and the CRF(2a) receptor are both expressed in mammals [9,23-24,26,28,30,48]. Expression of the 397-amino acid CRF(2c) receptor has only been detected in limbic regions of the human central nervous system [48]. Splicing of the CRF2 receptor variants occurs at the extreme 5'-terminus of the receptor gene and reflects usage of different promoters in man. The hCRF2 gene, which is located on chromosome 7p14-15, is ñ50 kb in size and contains 15 exons [14]. The first four exons give rise to the different 5'-ends of the splice variants hCRF2(a), hCRF2(b) and hCRF2(c), respectively; exons 5-15 form the common parts of the various hCRF2 splice variants. Exons 1 and 2, which are separated by ñ10.5 kb intronic sequence encode the CRF2(b)-specific part, followed by the CRF2(c) and CRF2(a) exons [14].
In rodents, CRF(2a) receptor mRNA is expressed primarily in brain neurons while CRF(2b) receptor mRNA is detected in non-neuronal brain structures and peripheral tissues [11]. The CRF(2a) receptor is the dominant CRF2 receptor splice variant expressed in the mammalian brain and is mainly found in areas that are critically involved in the mediation of stress responses [3,29]. In humans and tree shrews, CRF(2a) receptor mRNA expression is broader than in rodent brain [24,27]. Because CRF(2b) receptor mRNA can be detected in neuronal structures the distribution of these two splice variants may overlap in primates and primate-like animals. In the periphery, substantial expression of CRF2 receptor can be found in the heart, skeletal muscle, vasculature, and gastrointestinal tract. The CRF2(a) receptor is the major splice variant found in the peripheral tissues of humans [28,48], while the CRF2(b) splice variant is the CRF2 receptor peripherally expressed in rodents. The main expression sites for the rodent CRF(2b) receptor have been reported for heart, lung, skeletal muscle, gastrointestinal tract, testis and ovaries [11,24].
Pharmacological characterization of the CRF2 receptor splice variants revealed no major differences between CRF(2a), CRF(2b) and CRF(2c) receptors [16,24,47-48]. However, the binding profiles of these three CRF2 receptors strongly diverge from the binding profile of the CRF1 receptor [13,16,21,26,39,42]. The non-mammalian CRF peptides urotensin I and sauvagine and the mammalian peptides urocortin 1, urocortin 2 and urocortin 3 generally bind with up to 100-fold higher affinities to the CRF2 receptor than species homologues of CRF. In agreement with the binding data, a similar rank order of potency is typically observed when stimulation of intracellular cyclic AMP accumulation is measured [13,16,21,26,42]. Therefore, current evidence suggests that urocortin 2 and urocortin 3 represent the endogenous ligands for mammalian CRF2 receptor variants. Urocortin 1 is an endogenous ligand with equal potencies in activating CRF1 and CRF2 receptors.
Unsolved issues
Recently a third CRF receptor, termed CRF3, was cloned from catfish [1]. The catfish CRF[18] receptor was not detected in salmon [40]. The catfish CRF1 and CRF3 receptors are highly homologous. Interestingly, CRF3 receptor expression is restricted to the catfish pituitary, a tissue that normally expresses CRF1 receptors in other species [1]. Species homologues for this receptor may not exist.
Human urocortin 2 lacks the standard consensus site required for proteolytic cleavage and C-terminal amidation, which is a prerequisite for biological potency (reviewed in [10]). Therefore, human urocortin 2 may not be processed into a biologically active peptide in vivo [21,26]. The isolation of the urocortin 2 peptide from native human tissues would resolve this issue. In literature, the abbreviation UCN is frequently used for urocortins. Because the abbreviation CRF exists a similar abbreviation for the urocortins is currently discussed.
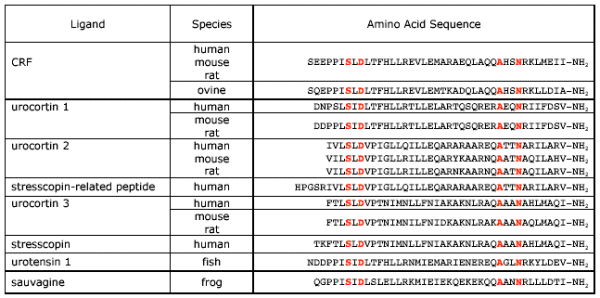
Table 1: Human, rat and mouse versions of CRF, urocortin 1, -2 and -3 are compared with human stresscopin, stresscopin-related peptide (extended versions of human urocortin 3 and -2) and fish urotensin I and frog sauvagine. The amino acids highlighted are conserved among all peptides.
References
1. Arai M, Assil IQ, Abou-Samra AB. (2001) Characterization of three corticotropin-releasing factor receptors in catfish: a novel third receptor is predominantly expressed in pituitary and urophysis. Endocrinology, 142 (1): 446-54. [PMID:11145609]
2. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. (1999) The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol, 160 (1): 1-12. [PMID:9854171]
3. Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. (1999) Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol, 415 (3): 285-312. [PMID:10553117]
4. Cepoi D, Sutton S, Arias C, Sawchenko P, Vale WW. (1999) Ovine genomic urocortin: cloning, pharmacologic characterization, and distribution of central mRNA. Brain Res Mol Brain Res, 68 (1-2): 109-18. [PMID:10320788]
5. Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. (1996) Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci, 17 (4): 166-72. [PMID:8984745]
6. Chang CP, Pearse 2nd RV, O'Connell S, Rosenfeld MG. (1993) Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron, 11 (6): 1187-95. [PMID:8274282]
7. Chen R, Lewis KA, Perrin MH, Vale WW. (1993) Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA, 90 (19): 8967-71. [PMID:7692441]
8. Dautzenberg FM, Dietrich K, Palchaudhuri MR, Spiess J. (1997) Identification of two corticotropin-releasing factor receptors from Xenopus laevis with high ligand selectivity: unusual pharmacology of the type 1 receptor. J Neurochem, 69 (4): 1640-9. [PMID:9326293]
9. Dautzenberg FM, Gutknecht E, Van der Linden I, Olivares-Reyes JA, Dürrenberger F, Hauger RL. (2004) Cell type specific calcium signaling by corticotropin-releasing factor type 1 (CRF1) and 2a (CRF2(a)) receptors: Gq coupling in human embryonic kidney 293 but not SK-N-MC neuroblastoma cells. Biochem Pharmacol, 68: 1833-1844. [PMID:15450949]
10. Dautzenberg FM, Hauger RL. (2002) The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci, 23: 71-77. [PMID:11830263]
11. Dautzenberg FM, Huber G, Higelin J, Py-Lang G, Kilpatrick GJ. (2000) Evidence for the abundant expression of arginine 185 containing human CRF(2alpha) receptors and the role of position 185 for receptor-ligand selectivity. Neuropharmacology, 39 (8): 1368-76. [PMID:10818253]
12. Dautzenberg FM, Kilpatrick GJ, Hauger RL, Moreau J. (2001) Molecular biology of the CRH receptors-- in the mood. Peptides, 22 (5): 753-60. [PMID:11337088]
13. Dautzenberg FM, Py-Lang G, Higelin J, Fischer C, Wright MB, Huber G. (2001) Different binding modes of amphibian and human corticotropin-releasing factor type 1 and type 2 receptors: evidence for evolutionary differences. J Pharmacol Exp Ther, 296 (1): 113-20. [PMID:11123370]
14. de Groef B, Grommen SV, Mertens I, Schoofs L, Kühn ER, Darras VM. (2004) Cloning and tissue distribution of the chicken type 2 corticotropin-releasing hormone receptor. Gen Comp Endocrinol, 138 (1): 89-95. [PMID:15242755]
15. De Souza EB, Perrin MH, Insel TR, Rivier J, Vale WW, Kuhar MJ. (1984) Corticotropin-releasing factor receptors in rat forebrain: autoradiographic identification. Science, 224: 1449-1451. [PMID:6328656]
16. Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE, Vaughan JM, Vale WW. (1996) Cloning and characterization of human urocortin. Endocrinology, 137 (5): 2167-70. [PMID:8612563]
17. Dunn AJ, Berridge CW. (1990) Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses?. Brain Res Brain Res Rev, 15 (2): 71-100. [PMID:1980834]
18. Hauger RL, Dautzenberg FM. (1999) Regulation of the stress response by corticotropin-releasing factor receptors. In Physiology and Medicine Edited by Conn PM, Freedman ME (Humana Press Inc.) 261-286. [ISBN:0896037250]
19. Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. (2003) International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev, 55 (1): 21-6. [PMID:12615952]
20. Heinrichs SC, De Souza EB. (1999) Corticotropin-releasing factor antagonists, binding-protein and receptors: implications for central nervous system disorders. Baillieres Best Pract Res Clin Endocrinol Metab, 13 (4): 541-54. [PMID:10903813]
21. Hsu SY, Hsueh AJ. (2001) Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med, 7 (5): 605-11. [PMID:11329063]
22. Kageyama K, Bradbury MJ, Zhao L, Blount AL, Vale WW. (1999) Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology, 140 (12): 5651-8. [PMID:10579329]
23. Kishimoto T, Pearse 2nd RV, Lin CR, Rosenfeld MG. (1995) A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci USA, 92 (4): 1108-12. [PMID:7755719]
24. Kostich WA, Chen A, Sperle K, Largent BL. (1998) Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol, 12 (8): 1077-85. [PMID:9717834]
25. Lederis K, Letter A, McMaster D, Moore G, Schlesinger D. (1982) Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science, 218 (4568): 162-5. [PMID:6981844]
26. Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W et al.. (2001) Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA, 98 (13): 7570-5. [PMID:11416224]
27. Li C, Vaughan J, Sawchenko PE, Vale WW. (2002) Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci, 22 (3): 991-1001. [PMID:11826127]
28. Liaw CW, Lovenberg TW, Barry G, Oltersdorf T, Grigoriadis DE, de Souza EB. (1996) Cloning and characterization of the human corticotropin-releasing factor-2 receptor complementary deoxyribonucleic acid. Endocrinology, 137 (1): 72-7. [PMID:8536644]
29. Lovenberg TW, Chalmers DT, Liu C, De Souza EB. (1995) CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology, 136: 4139-4142. [PMID:7544278]
30. Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. (1995) Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA, 92 (3): 836-40. [PMID:7846062]
31. Montecucchi PC, Henschen A. (1981) Amino acid composition and sequence analysis of sauvagine, a new active peptide from the skin of Phyllomedusa sauvagei. Int J Pept Protein Res, 18 (2): 113-20. [PMID:7309372]
32. Myers DA, Trinh JV, Myers TR. (1998) Structure and function of the ovine type 1 corticotropin releasing factor receptor (CRF1) and a carboxyl-terminal variant. Mol Cell Endocrinol, 144 (1-2): 21-35. [PMID:9863624]
33. Nappi RE, Rivest S. (1995) Stress-induced genetic expression of a selective corticotropin-releasing factor-receptor subtype within the rat ovaries: an effect dependent on the ovulatory cycle. Biol Reprod, 53 (6): 1417-28. [PMID:8562699]
34. Okawara Y, Morley SD, Burzio LO, Zwiers H, Lederis K, Richter D. (1988) Cloning and sequence analysis of cDNA for corticotropin-releasing factor precursor from the teleost fish Catostomus commersoni. Proc Natl Acad Sci USA, 85 (22): 8439-43. [PMID:3186733]
35. Oshida Y, Ikeda Y, Chaki S, Okuyama S. (2004) Monkey corticotropin-releasing factor1 receptor: Complementary DNA cloning and pharmacological characterization. Life Sci, 74: 1911-1924. [PMID:14761672]
36. Palchaudhuri MR, Hauger RL, Wille S, Fuchs E, Dautzenberg FM. (1999) Isolation and pharmacological characterization of two functional splice variants of corticotropin-releasing factor type 2 receptor from Tupaia belangeri. J Neuroendocrinol, 11 (6): 419-28. [PMID:10336722]
37. Palchaudhuri MR, Wille S, Mevenkamp G, Spiess J, Fuchs E, Dautzenberg FM. (1998) Corticotropin-releasing factor receptor type 1 from Tupaia belangeri--cloning, functional expression and tissue distribution. Eur J Biochem, 258 (1): 78-84. [PMID:9851694]
38. Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. (1993) Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology, 133 (6): 3058-61. [PMID:8243338]
39. Perrin MH, Sutton SW, Cervini LA, Rivier JE, Vale WW. (1999) Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. J Pharmacol Exp Ther, 288 (2): 729-34. [PMID:9918582]
40. Pohl S, Darlison MG, Clarke WC, Lederis K, Richter D. (2001) Cloning and functional pharmacology of two corticotropin-releasing factor receptors from a teleost fish. Eur J Pharmacol, 430 (2-3): 193-202. [PMID:11711031]
41. Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. (1994) Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA, 91 (19): 8777-81. [PMID:8090722]
42. Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW et al.. (2001) Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA, 98 (5): 2843-8. [PMID:11226328]
43. Smagin GN, Heinrichs SC, Dunn AJ. (2001) The role of CRH in behavioral responses to stress. Peptides, 22 (5): 713-24. [PMID:11337084]
44. Stenzel P, Kesterson R, Yeung W, Cone RD, Rittenberg MB, Stenzel-Poore MP. (1995) Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol Endocrinol, 9 (5): 637-45. [PMID:7565810]
45. Stenzel-Poore MP, Heldwein KA, Stenzel P, Lee S, Vale WW. (1992) Characterization of the genomic corticotropin-releasing factor (CRF) gene from Xenopus laevis: two members of the CRF family exist in amphibians. Mol Endocrinol, 6 (10): 1716-24. [PMID:1448118]
46. Swanson LW, Sawchenko PE, Rivier J, Vale WW. (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology, 36 (3): 165-86. [PMID:6601247]
47. Sánchez MM, Young LJ, Plotsky PM, Insel TR. (1999) Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol, 408 (3): 365-77. [PMID:10340512]
48. Valdenaire O, Giller T, Breu V, Gottowik J, Kilpatrick G. (1997) A new functional isoform of the human CRF2 receptor for corticotropin-releasing factor. Biochim Biophys Acta, 1352 (2): 129-32. [PMID:9199241]
49. Vale W, Spiess J, Rivier C, Rivier J. (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science, 213 (4514): 1394-7. [PMID:6267699]
50. Vale W, Vaughan J, Perrin M. (1997) Corticotropin-releasing factor (CRF) family of ligands and their receptors. The Endocrinoligist, 7: S3-S9.
51. Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C et al.. (1995) Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature, 378 (6554): 287-92. [PMID:7477349]
52. Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, Le Fur G, Caput D, Ferrara P. (1993) Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett, 335 (1): 1-5. [PMID:8243652]
53. Wynn PC, Hauger RL, Holmes MC, Millan MA, Catt KJ, Aguilera G. (1984) Brain and pituitary receptors for corticotropin releasing factor: localization and differential regulation after adrenalectomy. Peptides, 5: 1077-1084. [PMID:6099558]
54. Yu J, Xie LY, Abou-Samra AB. (1996) Molecular cloning of a type A chicken corticotropin-releasing factor receptor with high affinity for urotensin I. Endocrinology, 137 (1): 192-7. [PMID:8536612]
55. Zhao L, Donaldson CJ, Smith GW, Vale WW. (1998) The structures of the mouse and human urocortin genes (Ucn and UCN). Genomics, 50 (1): 23-33. [PMID:9628819]






