GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Enzyme Reaction
- Inhibitors
- Other Binding Ligands
- Large-scale Ligand Screening Data
- Immunopharmacology Comments
- Immuno Cell Type Associations
- Clinically-Relevant Mutations and Pathophysiology
- General Comments
- References
- How to cite this page
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 659 | Xq22.1 | BTK | Bruton tyrosine kinase | |
| Mouse | - | 659 | X 56.18 cM | Btk | Bruton agammaglobulinemia tyrosine kinase | |
| Rat | - | 660 | X q34 | Btk | Bruton tyrosine kinase | |
Previous and Unofficial Names  |
| AGMX1 | ATK | B-cell progenitor kinase | IMD1 | PSCTK1 | xid | X-linked immune deficiency | Bruton agammaglobulinemia tyrosine kinase |
Database Links  |
|
| Alphafold | Q06187 (Hs), P35991 (Mm) |
| BRENDA | 2.7.10.2 |
| CATH/Gene3D | 2.30.29.30, 3.30.505.10 |
| ChEMBL Target | CHEMBL5251 (Hs), CHEMBL3259478 (Mm) |
| Ensembl Gene | ENSG00000010671 (Hs), ENSMUSG00000031264 (Mm), ENSRNOG00000052407 (Rn) |
| Entrez Gene | 695 (Hs), 12229 (Mm), 367901 (Rn) |
| Human Protein Atlas | ENSG00000010671 (Hs) |
| KEGG Enzyme | 2.7.10.2 |
| KEGG Gene | hsa:695 (Hs), mmu:12229 (Mm), rno:367901 (Rn) |
| OMIM | 300300 (Hs) |
| Orphanet | ORPHA119094 (Hs) |
| Pharos | Q06187 (Hs) |
| RefSeq Nucleotide | NM_000061 (Hs), NM_013482 (Mm), NM_001007798 (Rn) |
| RefSeq Protein | NP_000052 (Hs), NP_038510 (Mm), NP_001007799 (Rn) |
| SynPHARM |
84961 (in complex with BMS-986142) 81122 (in complex with CGI1746) 83743 (in complex with GDC-0834) 84487 (in complex with ibrutinib) |
| UniProtKB | Q06187 (Hs), P35991 (Mm) |
| Wikipedia | BTK (Hs) |
Selected 3D Structures  |
|||||||||||||
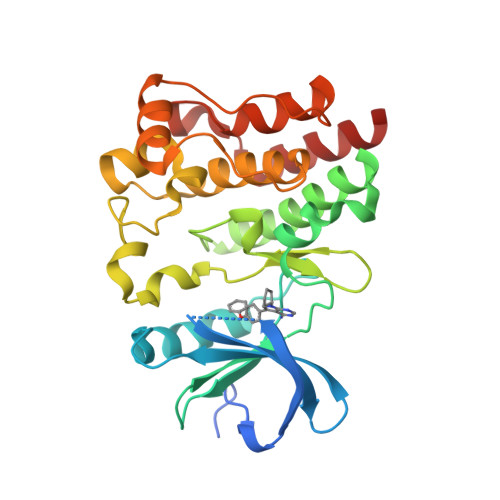
|
|
||||||||||||
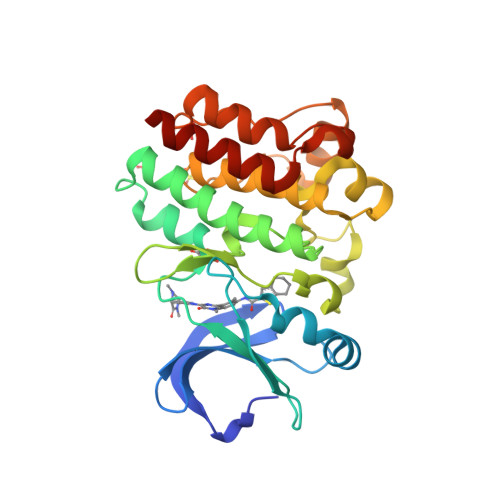
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BI705564 (Boehringer Ingelheim) is a selective, covalent and potent (nM) inhibitor of BTK. It was in early stage clinical stage development, but the first study in patients with systemic lupus erythematosus (NCT03771885) was withdrawn before recruitment began. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Binding Ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 15,56 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: BTK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: 1,20 |
 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: BTK/BTK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| The TEC family protein tyrosine kinases have been identified as key components of T-cell-receptor activation and signalling. TEC family kinases are expressed predominantly by haematopoietic cells. T cells express ITK, TXK and TEC [5]. BTK is expressed by all hematopoietic cells, except T cells and differentiated plasma cells. As BTK is an important mediator of critical B-cell pro-survival mechanisms including apoptosis, cell adhesion, and migration, it has been identified as a drug target for pharmaceutical intervention in B cell malignancies. BTK is the target of several small molecule tyrosine kinase inhibitors, including the approved anti-leukemia drug ibrutinib and several others in the development pipeline. BTK also plays an essential role in B cell receptor (BCR)-mediated signaling, Fcγ receptor signaling in monocytes, and Fcε receptor signaling in mast cells and basophils. All of these functions have been implicated in the pathophysiology of autoimmune disease, making BTK inhibition a valid strategy for the management of autoimmune diseases. BTK directly interacts with the NLRP3 inflammasome and modifies a number of NLRP3 tyrosine residues, activities that have a significant positive regulatory effect on NLRP3-mediated inflammatory responses [6]. |
| Cell Type Associations | ||||||
|
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| General Comments |
| The history, and pros and cons of the various small molecule BTK inhibitor classes that have been developed for clinical applications, and prospects for future drug design are reviewed by Liang et al. (2018) [39]. |
References
1. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
2. Angst D, Gessier F, Vulpetti A. (2015) Novel amino pyrimidine derivatives. Patent number: WO2015079417A1. Assignee: Novartis Ag. Priority date: 29/11/2013. Publication date: 04/06/2015.
3. Barf T, Kaptein A. (2012) Irreversible protein kinase inhibitors: balancing the benefits and risks. J Med Chem, 55 (14): 6243-62. [PMID:22621397]
4. Barf TA, Jans CGJM, Man PADeA, Oubrie AA, Raaijmakers HCA, Rewinkel JBM, Sterrenburg J-G, Wijkmans JCHM. (2014) 4-imidazopyridazin-1-yl-benzamides and 4-imidazotriazin-1-yl-benzamides as btk inhibitors. Patent number: US20140155385 A1. Assignee: Barf TA, Jans CGJM, Man PADeA, Oubrie AA, Raaijmakers HCA, Rewinkel JBM, Sterrenburg J-G, Wijkmans JCHM. Priority date: 19/07/2011. Publication date: 05/06/2014.
5. Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. (2005) Tec family kinases in T lymphocyte development and function. Annu Rev Immunol, 23: 549-600. [PMID:15771581]
6. Bittner ZA, Liu X, Mateo Tortola M, Tapia-Abellán A, Shankar S, Andreeva L, Mangan M, Spalinger M, Kalbacher H, Düwell P et al.. (2021) BTK operates a phospho-tyrosine switch to regulate NLRP3 inflammasome activity. J Exp Med, 218 (11). DOI: 10.1084/jem.20201656 [PMID:34554188]
7. Bonafoux D, Davis HM, Frank KE, Friedman MM, Herold JM, Hoemann MZ, Huntley R, Osuma A, Sheppard G, Somal GK et al.. (2014) Primary carboxamides as BTK inhibitors. Patent number: WO2014210255A1. Assignee: Abbvie Inc.. Priority date: 26/06/2013. Publication date: 31/12/2014.
8. Bradshaw JM, McFarland JM, Paavilainen VO, Bisconte A, Tam D, Phan VT, Romanov S, Finkle D, Shu J, Patel V et al.. (2015) Prolonged and tunable residence time using reversible covalent kinase inhibitors. Nat Chem Biol, 11 (7): 525-31. [PMID:26006010]
9. Brandhuber BJ, Brent LT, Eary CT, Kenna A, Khan F, Renshaw VFH, Spencer SR. (2020) Spray-dried dispersions and formulations of (s)-5-amino-3-(4-((5-fluoro-2-methoxybenzamido)methyl)phenyl)-1-(1,1,1-trifluoro propan-2-yl)-1h-pyrazole-4-carboxamide. Patent number: WO2020028258A1. Assignee: Loxo Oncology, Inc.. Priority date: 31/07/2018. Publication date: 06/02/2020.
10. Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, Chaves J, Wierda WG, Awan FT, Brown JR et al.. (2016) Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med, 374 (4): 323-32. [PMID:26641137]
11. Cha MY, Kang SJ, Kim MR, Lee JY, Jeon JY, Jo MG, Kwak EJ, Lee KO, Ha TH, Suh KH, Kim MS. (2011) Novel fused pyrimidine derivatives for inhd3ition of tyrosine kinase activity. Patent number: WO2011162515A2. Assignee: Hanmi Holdings Co. , Ltd.. Priority date: 23/06/2010. Publication date: 29/12/2011.
12. Chen X, Gao Y, Liu C, Ni H, Mulvihill M. (2015) Substituted nicotinimide inhibitors of btk and their preparation and use in the treatment of cancer, inflammation and autoimmune disease. Patent number: WO2015048662A2. Assignee: X-Rx Discovery, Inc.. Priority date: 03/09/2013. Publication date: 02/04/2015.
13. Coburn CA, Kumar DV, Lopez LA, Buzard DJ. (2021) Kinase inhibitors. Patent number: WO2021207549A1. Assignee: Gb005, Inc.. Priority date: 08/04/2021. Publication date: 14/10/2021.
14. Crawford JJ, Ortwine DF, Wei B, Young WB. (2013) Heteroaryl pyridone and aza-pyridone compounds as inhibitors of btk activity. Patent number: WO2013067274. Assignee: Genentech, Inc.. Priority date: 03/11/2011. Publication date: 10/05/2013.
15. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
16. Di Paolo JA, Huang T, Balazs M, Barbosa J, Barck KH, Bravo BJ, Carano RA, Darrow J, Davies DR, DeForge LE et al.. (2011) Specific Btk inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat Chem Biol, 7 (1): 41-50. [PMID:21113169]
17. Dobrovolsky D, Wang ES, Morrow S, Leahy C, Faust T, Nowak RP, Donovan KA, Yang G, Li Z, Fischer ES et al.. (2019) Bruton tyrosine kinase degradation as a therapeutic strategy for cancer. Blood, 133 (9): 952-961. [PMID:30545835]
18. Doyle SL, Jefferies CA, Feighery C, O'Neill LA. (2007) Signaling by Toll-like receptors 8 and 9 requires Bruton's tyrosine kinase. J Biol Chem, 282 (51): 36953-60. [PMID:17932028]
19. Evans E, Tester R, Aslanian S, Mazdiyasn H, Ponader S, Tesar B, Chaturvedi P, Nacht M, Stiede K, Witowski S et al.. Clinical Development of AVL - 292: A Potent, Selective Covalent Btk Inhibitor for the Treatment of B Cell Malignancies. Accessed on 29/10/2014. Modified on 29/10/2014. http://www.celgene.com, http://www.celgene.com/content/uploads/pdf/2011_ASH_AVL-292.pdf
20. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013) A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362]
21. Ghoshdastidar K, Patel H, Bhayani H, Patel A, Thakkar K, Patel D, Sharma M, Singh J, Mohapatra J, Chatterjee A et al.. (2020) ZYBT1, a potent, irreversible Bruton's Tyrosine Kinase (BTK) inhibitor that inhibits the C481S BTK with profound efficacy against arthritis and cancer. Pharmacol Res Perspect, 8 (4): e00565. [PMID:32790160]
22. Goldstein D, Owens TD. (2016) Tyrosine kinase inhibitors. Patent number: WO2016196840A1. Assignee: Principia Biopharma Inc.. Priority date: 03/06/2015. Publication date: 08/12/2016.
23. Goldstein DM. (2015) Tyrosine kinase inhibitors. Patent number: US9090621B2. Assignee: Principia Biopharma Inc. Priority date: 09/04/2013. Publication date: 28/07/2015.
24. Himmelbauer MK, Bajrami B, Basile R, Capacci A, Chen T, Choi CK, Gilfillan R, Gonzalez-Lopez de Turiso F, Gu C, Hoemberger M et al.. (2024) Discovery and Preclinical Characterization of BIIB129, a Covalent, Selective, and Brain-Penetrant BTK Inhibitor for the Treatment of Multiple Sclerosis. J Med Chem, 67 (10): 8122-8140. [PMID:38712838]
25. Hodous B, Liu-Bujalski L, Jones R, Bankston D, Johnson TL, Mochalkin I, Nguyen N, Qiu H, Goutopoulos A, Brugger N. (2012) Compositions and methods for the production of pyrimidine and pyridine compounds with btk inhibitory activity. Patent number: WO2012170976. Assignee: Merck Patent Gmbh. Priority date: 10/06/2011. Publication date: 13/12/2012.
26. Hong YR, Na JE, Min IS, Cha HJ, Kwon SK, Ro S, Cho JM. (2017) 2,3-dihydro-isoindole-1-on derivative as BTK kinase suppressant, and pharmaceutical composition including same. Patent number: US9758508B2. Assignee: CrystalGenomics Inc. Priority date: 28/12/2012. Publication date: 12/09/2017.
27. Hopkins BT, Bame E, Bajrami B, Black C, Bohnert T, Boiselle C, Burdette D, Burns JC, Delva L, Donaldson D et al.. (2022) Discovery and Preclinical Characterization of BIIB091, a Reversible, Selective BTK Inhibitor for the Treatment of Multiple Sclerosis. J Med Chem, 65 (2): 1206-1224. [PMID:34734694]
28. Hopkins BT, Conlon P, Chan TR, Jenkins TJ, Cai X, Humora M, Shi X, Miller RA, Thompson A. (2013) Pyrimidinyl tyrosine kinase inhibitors. Patent number: WO2013185084A1. Assignee: Biogen Idec Ma Inc., Sunesis Pharmaceuticals, Inc.. Priority date: 08/06/2012. Publication date: 12/12/2013.
29. Hopkins BT, Ma B, Prince R, Marx I, Lyssikatos JP, Zheng F, Peterson M, Patience DB. (2018) Benzoazepine analogs as inhibiting agents for bruton's tyrosine kinase. Patent number: WO2018191577A1. Assignee: Biogen Ma Inc.. Priority date: 14/04/2017. Publication date: 18/10/2018.
30. Horwood NJ, Page TH, McDaid JP, Palmer CD, Campbell J, Mahon T, Brennan FM, Webster D, Foxwell BM. (2006) Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol, 176 (6): 3635-41. [PMID:16517732]
31. Huang J, Ma Z, Peng X, Yang Z, Wu Y, Zhong G, Ouyang T, Chen Z, Liu Y, Wang Q et al.. (2024) Discovery of Novel Potent and Fast BTK PROTACs for the Treatment of Osteoclasts-Related Inflammatory Diseases. J Med Chem, 67 (4): 2438-2465. [PMID:38321747]
32. Iguchi S, Hosoi F, Sagara T. (2017) Fused pyrimidine compound or salt thereof. Patent number: US9580432B2. Assignee: Taiho Pharmaceutical Co Ltd. Priority date: 11/08/2014. Publication date: 28/02/2017.
33. Kawahata W, Asami T, Kiyoi T, Irie T, Kashimoto S, Furuichi H, Sawa M. (2021) Discovery of AS-1763: A Potent, Selective, Noncovalent, and Orally Available Inhibitor of Bruton's Tyrosine Kinase. J Med Chem, 64 (19): 14129-14141. [PMID:34529443]
34. Kawahata W, Asami T, Kiyoi T, Irie T, Taniguchi H, Asamitsu Y, Inoue T, Miyake T, Sawa M. (2018) Design and Synthesis of Novel Amino-triazine Analogues as Selective Bruton's Tyrosine Kinase Inhibitors for Treatment of Rheumatoid Arthritis. J Med Chem, 61 (19): 8917-8933. [PMID:30216722]
35. Kim KH, Maderna A, Schnute ME, Hegen M, Mohan S, Miyashiro J, Lin L, Li E, Keegan S, Lussier J et al.. (2011) Imidazo[1,5-a]quinoxalines as irreversible BTK inhibitors for the treatment of rheumatoid arthritis. Bioorg Med Chem Lett, 21 (21): 6258-63. [PMID:21958547]
36. Li X, Zuo Y, Tang G, Wang Y, Zhou Y, Wang X, Guo T, Xia M, Ding N, Pan Z. (2014) Discovery of a series of 2,5-diaminopyrimidine covalent irreversible inhibitors of Bruton's tyrosine kinase with in vivo antitumor activity. J Med Chem, 57 (12): 5112-28. [PMID:24915291]
37. Li YQ, Lannigan WG, Davoodi S, Daryaee F, Corrionero A, Alfonso P, Rodriguez-Santamaria JA, Wang N, Haley JD, Tonge PJ. (2023) Discovery of Novel Bruton’s Tyrosine Kinase PROTACs with Enhanced Selectivity and Cellular Efficacy. Journal of Mediconal Chemistry,. DOI: 10.1021/acs.jmedchem.3c00176
38. Li Z, Zou H, Zhu W, Shen C, Wang R, Liu W, Chen X, Tsui H, Yang Z, Zhang X. (2019) Erbb/btk inhibitors. Patent number: WO2019149164A1. Assignee: Dizal (Jiangsu) Pharmaceutical Co., Ltd. Priority date: 31/01/2018. Publication date: 08/09/2019.
39. Liang C, Tian D, Ren X, Ding S, Jia M, Xin M, Thareja S. (2018) The development of Bruton's tyrosine kinase (BTK) inhibitors from 2012 to 2017: A mini-review. Eur J Med Chem, 151: 315-326. [PMID:29631132]
40. Liu Q, Watterson SH, Batt DG, Ahmad S, Bertrand MB, Gong H, Guo W, Macor JE, Ngu K, Tebben J, Tino A. (2016) Indole carboxamide compounds. Patent number: US20160115126A1. Assignee: Bristol-Myers Squibb Co. Priority date: 24/10/2014. Publication date: 28/04/2016.
41. Lou Y, Owens TD, Kuglstatter A, Kondru RK, Goldstein DM. (2012) Bruton's tyrosine kinase inhibitors: approaches to potent and selective inhibition, preclinical and clinical evaluation for inflammatory diseases and B cell malignancies. J Med Chem, 55 (10): 4539-50. [PMID:22394077]
42. Ma B, Bohnert T, Otipoby KL, Tien E, Arefayene M, Bai J, Bajrami B, Bame E, Chan TR, Humora M et al.. (2020) Discovery of BIIB068: A Selective, Potent, Reversible Bruton's Tyrosine Kinase Inhibitor as an Orally Efficacious Agent for Autoimmune Diseases. J Med Chem, 63 (21): 12526-12541. [PMID:32696648]
43. Mahajan S, Ghosh S, Sudbeck EA, Zheng Y, Downs S, Hupke M, Uckun FM. (1999) Rational design and synthesis of a novel anti-leukemic agent targeting Bruton's tyrosine kinase (BTK), LFM-A13 [alpha-cyano-beta-hydroxy-beta-methyl-N-(2, 5-dibromophenyl)propenamide]. J Biol Chem, 274 (14): 9587-99. [PMID:10092645]
44. Marcotte DJ, Liu YT, Arduini RM, Hession CA, Miatkowski K, Wildes CP, Cullen PF, Hong V, Hopkins BT, Mertsching E et al.. (2010) Structures of human Bruton's tyrosine kinase in active and inactive conformations suggest a mechanism of activation for TEC family kinases. Protein Sci, 19 (3): 429-39. [PMID:20052711]
45. Owens T, Verner E. (2014) PYRAZOLOPYRIMIDINE COMPOUNDS AS KINASE INHIBITORS. Patent number: WO2014039899. Assignee: Principia Biopharma. Priority date: 06/09/2013. Publication date: 13/03/2014.
46. Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC, Mendonca RV, Sweeney MD, Scott KC, Grothaus PG et al.. (2007) Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase. ChemMedChem, 2 (1): 58-61. [PMID:17154430]
47. Qiu H, Liu-Bujalski L, Caldwell RD, Follis AV, Gardberg A, Goutopoulos A, Grenningloh R, Head J, Johnson T, Jones R et al.. (2018) Discovery of potent, highly selective covalent irreversible BTK inhibitors from a fragment hit. Bioorg Med Chem Lett, 28 (17): 2939-2944. [PMID:30122225]
48. Reiff SD, Mantel R, Smith LL, Greene JT, Muhowski EM, Fabian CA, Goettl VM, Tran M, Harrington BK, Rogers KA et al.. (2018) The BTK Inhibitor ARQ 531 Targets Ibrutinib-Resistant CLL and Richter Transformation. Cancer Discov, 8 (10): 1300-1315. [PMID:30093506]
49. Robbins DW, Noviski MA, Tan YS, Konst ZA, Kelly A, Auger P, Brathaban N, Cass R, Chan ML, Cherala G et al.. (2024) Discovery and Preclinical Pharmacology of NX-2127, an Orally Bioavailable Degrader of Bruton's Tyrosine Kinase with Immunomodulatory Activity for the Treatment of Patients with B Cell Malignancies. J Med Chem, 67 (4): 2321-2336. [PMID:38300987]
50. Sabat M, Dougan DR, Knight B, Lawson JD, Scorah N, Smith CR, Taylor ER, Vu P, Wyrick C, Wang H et al.. (2021) Discovery of the Bruton's Tyrosine Kinase Inhibitor Clinical Candidate TAK-020 (S)-5-(1-((1-Acryloylpyrrolidin-3-yl)oxy)isoquinolin-3-yl)-2,4-dihydro-3H-1,2,4-triazol-3-one, by Fragment-Based Drug Design. J Med Chem, 64 (17): 12893-12902. [PMID:34448571]
51. Tichenor MS, Wiener JJM, Rao NL, Bacani GM, Wei J, Pooley Deckhut C, Barbay JK, Kreutter KD, Chang L, Clancy KW et al.. (2022) Discovery of JNJ-64264681: A Potent and Selective Covalent Inhibitor of Bruton's Tyrosine Kinase. J Med Chem, 65 (21): 14326-14336. [PMID:36314537]
52. Wang T, Lamb ML, Block MH, Davies AM, Han Y, Hoffmann E, Ioannidis S, Josey JA, Liu ZY, Lyne PD et al.. (2012) Discovery of Disubstituted Imidazo[4,5-b]pyridines and Purines as Potent TrkA Inhibitors. ACS Med Chem Lett, 3 (9): 705-9. [PMID:24900538]
53. Wang Z, Guo Y. (2016) Substituted pyrazolo[1,5-a]pyrimidines as bruton's tyrosine kinase modulators. Patent number: US9447106B2. Assignee: Beigene Ltd.. Priority date: 25/04/2013. Publication date: 20/09/2016.
54. Watterson SH, De Lucca GV, Shi Q, Langevine CM, Liu Q, Batt DG, Beaudoin Bertrand M, Gong H, Dai J, Yip S et al.. (2016) Discovery of 6-Fluoro-5-(R)-(3-(S)-(8-fluoro-1-methyl-2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)-2-methylphenyl)-2-(S)-(2-hydroxypropan-2-yl)-2,3,4,9-tetrahydro-1H-carbazole-8-carboxamide (BMS-986142): A Reversible Inhibitor of Bruton's Tyrosine Kinase (BTK) Conformationally Constrained by Two Locked Atropisomers. J Med Chem, 59 (19): 9173-9200. [PMID:27583770]
55. Watterson SH, Liu Q, Beaudoin Bertrand M, Batt DG, Li L, Pattoli MA, Skala S, Cheng L, Obermeier MT, Moore R et al.. (2019) Discovery of Branebrutinib (BMS-986195): A Strategy for Identifying a Highly Potent and Selective Covalent Inhibitor Providing Rapid in Vivo Inactivation of Bruton's Tyrosine Kinase (BTK). J Med Chem, 62 (7): 3228-3250. [PMID:30893553]
56. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
57. Wu H, Huang Q, Qi Z, Chen Y, Wang A, Chen C, Liang Q, Wang J, Chen W, Dong J et al.. (2017) Irreversible inhibition of BTK kinase by a novel highly selective inhibitor CHMFL-BTK-11 suppresses inflammatory response in rheumatoid arthritis model. Sci Rep, 7 (1): 466. [PMID:28352114]
58. Xu D, Kim Y, Postelnek J, Vu MD, Hu DQ, Liao C, Bradshaw M, Hsu J, Zhang J, Pashine A et al.. (2012) RN486, a selective Bruton's tyrosine kinase inhibitor, abrogates immune hypersensitivity responses and arthritis in rodents. J Pharmacol Exp Ther, 341 (1): 90-103. [PMID:22228807]
59. Xu X, Mao L, Xu W, Tang W, Zhang X, Xi B, Xu R, Fang X, Liu J, Fang C et al.. (2016) AC0010, an Irreversible EGFR Inhibitor Selectively Targeting Mutated EGFR and Overcoming T790M-Induced Resistance in Animal Models and Lung Cancer Patients. Mol Cancer Ther, 15 (11): 2586-2597. [PMID:27573423]
60. Yamamoto S, Yoshizawa T. (2011) Purinone derivative. Patent number: WO2011152351. Assignee: Ono Pharmaceutical Co., Ltd.. Priority date: 31/05/2010. Publication date: 08/12/2011.
61. Yao X, Sun X, Jin S, Yang L, Xu H, Rao Y. (2019) Discovery of 4-Aminoquinoline-3-carboxamide Derivatives as Potent Reversible Bruton's Tyrosine Kinase Inhibitors for the Treatment of Rheumatoid Arthritis. J Med Chem, 62 (14): 6561-6574. [PMID:31260299]
62. Young WB, Barbosa J, Blomgren P, Bremer MC, Crawford JJ, Dambach D, Gallion S, Hymowitz SG, Kropf JE, Lee SH et al.. (2015) Potent and selective Bruton's tyrosine kinase inhibitors: discovery of GDC-0834. Bioorg Med Chem Lett, 25 (6): 1333-7. [PMID:25701252]
63. Zhong Y, Dong S, Strattan E, Ren L, Butchar JP, Thornton K, Mishra A, Porcu P, Bradshaw JM, Bisconte A et al.. (2015) Targeting interleukin-2-inducible T-cell kinase (ITK) and resting lymphocyte kinase (RLK) using a novel covalent inhibitor PRN694. J Biol Chem, 290 (10): 5960-78. [PMID:25593320]
64. Zhou Q, Shen C, Chen X, Liu W, WAng R, Zeng Q, Tsui H, Yang Z, Zhang X. (2021) Btk inhibitors. Patent number: WO2021136219A1. Assignee: Dizal (Jiangsu) Pharmaceutical Co., Ltd.. Priority date: 29/12/2020. Publication date: 08/07/2021.
65. Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, Cortot AB, Chirieac L, Iacob RE, Padera R et al.. (2009) Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature, 462 (7276): 1070-4. [PMID:20033049]
How to cite this page
Tec family: Bruton tyrosine kinase. Last modified on 28/07/2025. Accessed on 26/02/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=1948.

 Target has curated data in GtoImmuPdb
Target has curated data in GtoImmuPdb









