Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Associated Proteins
- Functional Characteristics
- Ion Selectivity and Conductance
- Voltage Dependence
- Activators
- Inhibitors
- Gating Inhibitors
- Channel Blockers
- Tissue Distribution
- Physiological Functions
- Phenotypes, Alleles and Disease Models
- Clinically-Relevant Mutations and Pathophysiology
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
|||||||
| Species | TM | P Loops | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 24 | 1 | 1836 | 17q23.3 | SCN4A | sodium voltage-gated channel alpha subunit 4 | 2,11,67 |
| Mouse | 24 | 1 | 1841 | 11 68.91 cM | Scn4a | sodium channel, voltage-gated, type IV, alpha | 1,72 |
| Rat | 24 | 1 | 1840 | 10q32.1 | Scn4a | sodium voltage-gated channel alpha subunit 4 | 1,60 |
Database Links  |
|
| Alphafold | P35499 (Hs), Q9ER60 (Mm), P15390 (Rn) |
| ChEMBL Target | CHEMBL2072 (Hs), CHEMBL3616353 (Mm), CHEMBL3509 (Rn) |
| DrugBank Target | P35499 (Hs) |
| Ensembl Gene | ENSG00000007314 (Hs), ENSMUSG00000001027 (Mm), ENSRNOG00000012134 (Rn) |
| Entrez Gene | 6329 (Hs), 110880 (Mm), 25722 (Rn) |
| Human Protein Atlas | ENSG00000007314 (Hs) |
| KEGG Gene | hsa:6329 (Hs), mmu:110880 (Mm), rno:25722 (Rn) |
| OMIM | 603967 (Hs) |
| Orphanet | ORPHA118507 (Hs) |
| Pharos | P35499 (Hs) |
| RefSeq Nucleotide | NM_000334 (Hs), NM_133199 (Mm), NM_013178 (Rn) |
| RefSeq Protein | NP_000325 (Hs), NP_573462 (Mm), NP_037310 (Rn) |
| UniProtKB | P35499 (Hs), Q9ER60 (Mm), P15390 (Rn) |
| Wikipedia | SCN4A (Hs) |
Selected 3D Structures  |
|||||||||||
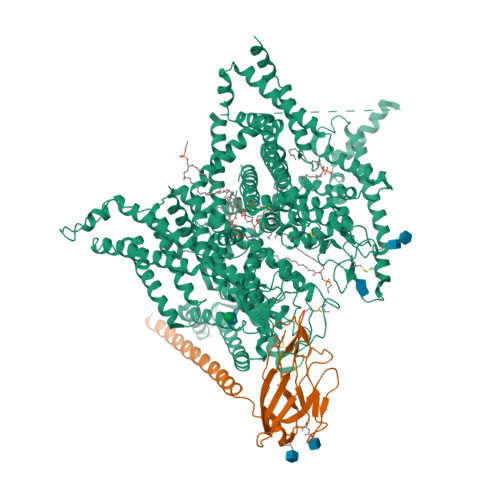
|
|
||||||||||
Associated Proteins  |
||||||||||||||||||||
|
|
|
||||||||||||||||||
Functional Characteristics  |
|
| Activation V0.5 = -30 mV. Fast inactivation (0.6 ms) | |
Ion Selectivity and Conductance  |
||||||
|
Voltage Dependence  |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
Download all structure-activity data for this target as a CSV file 
| Activators | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Gating inhibitors 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Channel Blockers | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific channel blocker tables | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tissue Distribution 
|
||||||||
|
||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
| Physiological Functions Comments | ||||||||
| The stated role of Nv1.4 has been reported for mamalian skeletal muscles [60-61]. | ||||||||
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinically-Relevant Mutations and Pathophysiology Comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The majority of Nav1.4 mutations in these diseases alter the inactivation properties of the channel leading to susceptability to periods of hyperactivity (causing myotonia) or inactivation (causing paralysis). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
1. Ambrose C, Cheng S, Fontaine B, Nadeau JH, MacDonald M, Gusella JF. (1992) The alpha-subunit of the skeletal muscle sodium channel is encoded proximal to Tk-1 on mouse chromosome 11. Mamm Genome, 3 (3): 151-5. [PMID:1352160]
2. Arnestad M, Crotti L, Rognum TO, Insolia R, Pedrazzini M, Ferrandi C, Vege A, Wang DW, Rhodes TE, George AL, Schwartz PJ. (2007) Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation, 115 (3): 361-7. [PMID:17210839]
3. Arzel-Hézode M, Sternberg D, Tabti N, Vicart S, Goizet C, Eymard B, Fontaine B, Fournier E. (2010) Homozygosity for dominant mutations increases severity of muscle channelopathies. Muscle Nerve, 41 (4): 470-7. [PMID:19882638]
4. Bendahhou S, Cummins TR, Kula RW, Fu YH, Ptácek LJ. (2002) Impairment of slow inactivation as a common mechanism for periodic paralysis in DIIS4-S5. Neurology, 58 (8): 1266-72. [PMID:11971097]
5. Bendahhou S, Cummins TR, Kwiecinski H, Waxman SG, Ptácek LJ. (1999) Characterization of a new sodium channel mutation at arginine 1448 associated with moderate Paramyotonia congenita in humans. J Physiol (Lond.), 518 ( Pt 2): 337-44. [PMID:10381583]
6. Bennett ES. (2004) Channel activation voltage alone is directly altered in an isoform-specific manner by Na(v1.4) and Na(v1.5) cytoplasmic linkers. J Membr Biol, 197 (3): 155-68. [PMID:15042347]
7. Bouhours M, Sternberg D, Davoine CS, Ferrer X, Willer JC, Fontaine B, Tabti N. (2004) Functional characterization and cold sensitivity of T1313A, a new mutation of the skeletal muscle sodium channel causing paramyotonia congenita in humans. J Physiol (Lond.), 554 (Pt 3): 635-47. [PMID:14617673]
8. Bulman DE, Scoggan KA, van Oene MD, Nicolle MW, Hahn AF, Tollar LL, Ebers GC. (1999) A novel sodium channel mutation in a family with hypokalemic periodic paralysis. Neurology, 53 (9): 1932-6. [PMID:10599760]
9. Cannon SC. (2002) An expanding view for the molecular basis of familial periodic paralysis. Neuromuscul Disord, 12 (6): 533-43. [PMID:12117476]
10. Carle T, Lhuillier L, Luce S, Sternberg D, Devuyst O, Fontaine B, Tabti N. (2006) Gating defects of a novel Na+ channel mutant causing hypokalemic periodic paralysis. Biochem Biophys Res Commun, 348 (2): 653-61. [PMID:16890191]
11. Chahine M, Bennett PB, George Jr AL, Horn R. (1994) Functional expression and properties of the human skeletal muscle sodium channel. Pflugers Arch, 427 (1-2): 136-42. [PMID:8058462]
12. Chahine M, Plante E, Kallen RG. (1996) Sea anemone toxin (ATX II) modulation of heart and skeletal muscle sodium channel alpha-subunits expressed in tsA201 cells. J Membr Biol, 152 (1): 39-48. [PMID:8660409]
13. Chen LQ, Chahine M, Kallen RG, Barchi RL, Horn R. (1992) Chimeric study of sodium channels from rat skeletal and cardiac muscle. FEBS Lett, 309 (3): 253-7. [PMID:1325372]
14. Cohen-Armon M, Sokolovsky M. (1993) Evidence for involvement of the voltage-dependent Na+ channel gating in depolarization-induced activation of G-proteins. J Biol Chem, 268 (13): 9824-38. [PMID:8387506]
15. Dice MS, Abbruzzese JL, Wheeler JT, Groome JR, Fujimoto E, Ruben PC. (2004) Temperature-sensitive defects in paramyotonia congenita mutants R1448C and T1313M. Muscle Nerve, 30 (3): 277-88. [PMID:15318338]
16. Ferrera L, Moran O. (2006) Beta1-subunit modulates the Nav1.4 sodium channel by changing the surface charge. Exp Brain Res, 172 (2): 139-50. [PMID:16432696]
17. Fleischhauer R, Mitrovic N, Deymeer F, Lehmann-Horn F, Lerche H. (1998) Effects of temperature and mexiletine on the F1473S Na+ channel mutation causing paramyotonia congenita. Pflugers Arch, 436 (5): 757-65. [PMID:9716710]
18. Gay S, Dupuis D, Faivre L, Masurel-Paulet A, Labenne M, Colombani M, Soichot P, Huet F, Hainque B, Sternberg D et al.. (2008) Severe neonatal non-dystrophic myotonia secondary to a novel mutation of the voltage-gated sodium channel (SCN4A) gene. Am J Med Genet A, 146 (3): 380-3. [PMID:18203179]
19. George AL. (2005) Inherited disorders of voltage-gated sodium channels. J Clin Invest, 115 (8): 1990-9. [PMID:16075039]
20. George Jr AL, Komisarof J, Kallen RG, Barchi RL. (1992) Primary structure of the adult human skeletal muscle voltage-dependent sodium channel. Ann Neurol, 31 (2): 131-7. [PMID:1315496]
21. Green DS, George AL, Cannon SC. (1998) Human sodium channel gating defects caused by missense mutations in S6 segments associated with myotonia: S804F and V1293I. J Physiol (Lond.), 510 ( Pt 3): 685-94. [PMID:9660885]
22. Groome JR, Fujimoto E, Ruben PC. (2005) K-aggravated myotonia mutations at residue G1306 differentially alter deactivation gating of human skeletal muscle sodium channels. Cell Mol Neurobiol, 25 (7): 1075-92. [PMID:16392038]
23. Heine R, Pika U, Lehmann-Horn F. (1993) A novel SCN4A mutation causing myotonia aggravated by cold and potassium. Hum Mol Genet, 2 (9): 1349-53. [PMID:8242056]
24. Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA. (1992) Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science, 256 (5058): 839-42. [PMID:1375395]
25. Jurkat-Rott K, Mitrovic N, Hang C, Kouzmekine A, Iaizzo P, Herzog J, Lerche H, Nicole S, Vale-Santos J, Chauveau D et al.. (2000) Voltage-sensor sodium channel mutations cause hypokalemic periodic paralysis type 2 by enhanced inactivation and reduced current. Proc Natl Acad Sci USA, 97 (17): 9549-54. [PMID:10944223]
26. Kimura T, Yamaoka K, Kinoshita E, Maejima H, Yuki T, Yakehiro M, Seyama I. (2001) Novel site on sodium channel alpha-subunit responsible for the differential sensitivity of grayanotoxin in skeletal and cardiac muscle. Mol Pharmacol, 60 (4): 865-72. [PMID:11562450]
27. Kondratiev A, Tomaselli GF. (2003) Altered gating and local anesthetic block mediated by residues in the I-S6 and II-S6 transmembrane segments of voltage-dependent Na+ channels. Mol Pharmacol, 64 (3): 741-52. [PMID:12920212]
28. Lee SC, Kim HS, Park YE, Choi YC, Park KH, Kim DS. (2009) Clinical Diversity of SCN4A-Mutation-Associated Skeletal Muscle Sodium Channelopathy. J Clin Neurol, 5 (4): 186-91. [PMID:20076800]
29. Lehmann-Horn F, Jurkat-Rott K. (1999) Voltage-gated ion channels and hereditary disease. Physiol Rev, 79 (4): 1317-72. [PMID:10508236]
30. Lehmann-Horn F, Rüdel R, Ricker K. (1993) Non-dystrophic myotonias and periodic paralyses. A European Neuromuscular Center Workshop held 4-6 October 1992, Ulm, Germany. Neuromuscul Disord, 3 (2): 161-8. [PMID:7689382]
31. Lerche H, Heine R, Pika U, George AL, Mitrovic N, Browatzki M, Weiss T, Rivet-Bastide M, Franke C, Lomonaco M. (1993) Human sodium channel myotonia: slowed channel inactivation due to substitutions for a glycine within the III-IV linker. J Physiol (Lond.), 470: 13-22. [PMID:8308722]
32. Lerche H, Mitrovic N, Dubowitz V, Lehmann-Horn F. (1996) Paramyotonia congenita: the R1448P Na+ channel mutation in adult human skeletal muscle. Ann Neurol, 39 (5): 599-608. [PMID:8619545]
33. Makielski JC, Limberis J, Fan Z, Kyle JW. (1999) Intrinsic lidocaine affinity for Na channels expressed in Xenopus oocytes depends on alpha (hH1 vs. rSkM1) and beta 1 subunits. Cardiovasc Res, 42 (2): 503-9. [PMID:10533585]
34. Marcotte P, Chen LQ, Kallen RG, Chahine M. (1997) Effects of Tityus serrulatus scorpion toxin gamma on voltage-gated Na+ channels. Circ Res, 80 (3): 363-9. [PMID:9048656]
35. Matthews E, Labrum R, Sweeney MG, Sud R, Haworth A, Chinnery PF, Meola G, Schorge S, Kullmann DM, Davis MB et al.. (2009) Voltage sensor charge loss accounts for most cases of hypokalemic periodic paralysis. Neurology, 72 (18): 1544-7. [PMID:19118277]
36. McClatchey AI, McKenna-Yasek D, Cros D, Worthen HG, Kuncl RW, DeSilva SM, Cornblath DR, Gusella JF, Brown RH. (1992) Novel mutations in families with unusual and variable disorders of the skeletal muscle sodium channel. Nat Genet, 2 (2): 148-52. [PMID:1338909]
37. McClatchey AI, Van den Bergh P, Pericak-Vance MA, Raskind W, Verellen C, McKenna-Yasek D, Rao K, Haines JL, Bird T, Brown Jr RH et al.. (1992) Temperature-sensitive mutations in the III-IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell, 68 (4): 769-74. [PMID:1310898]
38. McKerrall SJ, Nguyen T, Lai KW, Bergeron P, Deng L, DiPasquale A, Chang JH, Chen J, Chernov-Rogan T, Hackos DH et al.. (2019) Structure- and Ligand-Based Discovery of Chromane Arylsulfonamide Nav1.7 Inhibitors for the Treatment of Chronic Pain. J Med Chem, 62 (8): 4091-4109. [PMID:30943032]
39. McNulty MM, Hanck DA. (2004) State-dependent mibefradil block of Na+ channels. Mol Pharmacol, 66 (6): 1652-61. [PMID:15562257]
40. Miller TM, Dias da Silva MR, Miller HA, Kwiecinski H, Mendell JR, Tawil R, McManis P, Griggs RC, Angelini C, Servidei S, Petajan J, Dalakas MC, Ranum LP, Fu YH, Ptácek LJ. (2004) Correlating phenotype and genotype in the periodic paralyses. Neurology, 63 (9): 1647-55. [PMID:15534250]
41. Mitrović N, George AL, Lerche H, Wagner S, Fahlke C, Lehmann-Horn F. (1995) Different effects on gating of three myotonia-causing mutations in the inactivation gate of the human muscle sodium channel. J Physiol (Lond.), 487 ( Pt 1): 107-14. [PMID:7473241]
42. Okuda S, Kanda F, Nishimoto K, Sasaki R, Chihara K. (2001) Hyperkalemic periodic paralysis and paramyotonia congenita--a novel sodium channel mutation. J Neurol, 248 (11): 1003-4. [PMID:11757950]
43. Oliveira JS, Redaelli E, Zaharenko AJ, Cassulini RR, Konno K, Pimenta DC, Freitas JC, Clare JJ, Wanke E. (2004) Binding specificity of sea anemone toxins to Nav 1.1-1.6 sodium channels: unexpected contributions from differences in the IV/S3-S4 outer loop. J Biol Chem, 279 (32): 33323-35. [PMID:15169781]
44. Pan X, Li Z, Zhou Q, Shen H, Wu K, Huang X, Chen J, Zhang J, Zhu X, Lei J et al.. (2018) Structure of the human voltage-gated sodium channel Nav1.4 in complex with β1. Science, 362 (6412). [PMID:30190309]
45. Park YH, Kim JB. (2010) An atypical phenotype of hypokalemic periodic paralysis caused by a mutation in the sodium channel gene SCN4A. Korean J Pediatr, 53 (10): 909-12. [PMID:21189962]
46. Penzotti JL, Lipkind G, Fozzard HA, Dudley Jr SC. (2001) Specific neosaxitoxin interactions with the Na+ channel outer vestibule determined by mutant cycle analysis. Biophys J, 80 (2): 698-706. [PMID:11159437]
47. Petitprez S, Tiab L, Chen L, Kappeler L, Rösler KM, Schorderet D, Abriel H, Burgunder JM. (2008) A novel dominant mutation of the Nav1.4 alpha-subunit domain I leading to sodium channel myotonia. Neurology, 71 (21): 1669-75. [PMID:19015483]
48. Ptacek LJ, Gouw L, Kwieciński H, McManis P, Mendell JR, Barohn RJ, George Jr AL, Barchi RL, Robertson M, Leppert MF. (1993) Sodium channel mutations in paramyotonia congenita and hyperkalemic periodic paralysis. Ann Neurol, 33 (3): 300-7. [PMID:8388676]
49. Ptácek LJ, George Jr AL, Barchi RL, Griggs RC, Riggs JE, Robertson M, Leppert MF. (1992) Mutations in an S4 segment of the adult skeletal muscle sodium channel cause paramyotonia congenita. Neuron, 8 (5): 891-7. [PMID:1316765]
50. Ptácek LJ, George Jr AL, Griggs RC, Tawil R, Kallen RG, Barchi RL, Robertson M, Leppert MF. (1991) Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell, 67 (5): 1021-7. [PMID:1659948]
51. Ptáĉek LJ, Tawil R, Griggs RC, Meola G, McManis P, Barohn RJ, Mendell JR, Harris C, Spitzer R, Santiago F et al.. (1994) Sodium channel mutations in acetazolamide-responsive myotonia congenita, paramyotonia congenita, and hyperkalemic periodic paralysis. Neurology, 44 (8): 1500-3. [PMID:8058156]
52. Ricker K, Moxley RT, Heine R, Lehmann-Horn F. (1994) Myotonia fluctuans. A third type of muscle sodium channel disease. Arch Neurol, 51 (11): 1095-102. [PMID:7980103]
53. Rojas CV, Wang JZ, Schwartz LS, Hoffman EP, Powell BR, Brown Jr RH. (1991) A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature, 354 (6352): 387-9. [PMID:1659668]
54. Rosenbohm A, Rüdel R, Fahlke C. (1999) Regulation of the human skeletal muscle chloride channel hClC-1 by protein kinase C. J Physiol (Lond.), 514 ( Pt 3): 677-85. [PMID:9882739]
55. Safo P, Rosenbaum T, Shcherbatko A, Choi DY, Han E, Toledo-Aral JJ, Olivera BM, Brehm P, Mandel G. (2000) Distinction among neuronal subtypes of voltage-activated sodium channels by mu-conotoxin PIIIA. J Neurosci, 20 (1): 76-80. [PMID:10627583]
56. Sternberg D, Maisonobe T, Jurkat-Rott K, Nicole S, Launay E, Chauveau D, Tabti N, Lehmann-Horn F, Hainque B, Fontaine B. (2001) Hypokalaemic periodic paralysis type 2 caused by mutations at codon 672 in the muscle sodium channel gene SCN4A. Brain, 124 (Pt 6): 1091-9. [PMID:11353725]
57. Stunnenberg BC, Ginjaar HB, Trip J, Faber CG, van Engelen BG, Drost G. (2010) Isolated eyelid closure myotonia in two families with sodium channel myotonia. Neurogenetics, 11 (2): 257-60. [PMID:19876661]
58. Sun W, Cohen SA, Barchi RL. (1995) Localization of epitopes for monoclonal antibodies directed against the adult rat skeletal muscle sodium channel (rSkM1) using polymerase chain reaction, fusion proteins, and western blotting. Anal Biochem, 226 (1): 188-91. [PMID:7785772]
59. Tanaka JC, Eccleston JF, Barchi RL. (1983) Cation selectivity characteristics of the reconstituted voltage-dependent sodium channel purified from rat skeletal muscle sarcolemma. J Biol Chem, 258 (12): 7519-26. [PMID:6305944]
60. Trimmer JS, Cooperman SS, Agnew WS, Mandel G. (1990) Regulation of muscle sodium channel transcripts during development and in response to denervation. Dev Biol, 142 (2): 360-7. [PMID:2175278]
61. Trimmer JS, Cooperman SS, Tomiko SA, Zhou JY, Crean SM, Boyle MB, Kallen RG, Sheng ZH, Barchi RL, Sigworth FJ et al.. (1989) Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron, 3 (1): 33-49. [PMID:2559760]
62. Tsujino A, Maertens C, Ohno K, Shen XM, Fukuda T, Harper CM, Cannon SC, Engel AG. (2003) Myasthenic syndrome caused by mutation of the SCN4A sodium channel. Proc Natl Acad Sci USA, 100 (12): 7377-82. [PMID:12766226]
63. Vicart S, Sternberg D, Fournier E, Ochsner F, Laforet P, Kuntzer T, Eymard B, Hainque B, Fontaine B. (2004) New mutations of SCN4A cause a potassium-sensitive normokalemic periodic paralysis. Neurology, 63 (11): 2120-7. [PMID:15596759]
64. Wagner S, Lerche H, Mitrovic N, Heine R, George AL, Lehmann-Horn F. (1997) A novel sodium channel mutation causing a hyperkalemic paralytic and paramyotonic syndrome with variable clinical expressivity. Neurology, 49 (4): 1018-25. [PMID:9339683]
65. Wang GK, Russell C, Wang SY. (2004) Mexiletine block of wild-type and inactivation-deficient human skeletal muscle hNav1.4 Na+ channels. J Physiol (Lond.), 554 (Pt 3): 621-33. [PMID:14608007]
66. Wang GK, Wang SY. (2003) Veratridine block of rat skeletal muscle Nav1.4 sodium channels in the inner vestibule. J Physiol (Lond.), 548 (Pt 3): 667-75. [PMID:12626674]
67. Wang JZ, Rojas CV, Zhou JH, Schwartz LS, Nicholas H, Hoffman EP. (1992) Sequence and genomic structure of the human adult skeletal muscle sodium channel alpha subunit gene on 17q. Biochem Biophys Res Commun, 182 (2): 794-801. [PMID:1310396]
68. Wang SY, Barile M, Wang GK. (2001) A phenylalanine residue at segment D3-S6 in Nav1.4 voltage-gated Na(+) channels is critical for pyrethroid action. Mol Pharmacol, 60 (3): 620-8. [PMID:11502895]
69. Wang SY, Wang GK. (1998) Point mutations in segment I-S6 render voltage-gated Na+ channels resistant to batrachotoxin. Proc Natl Acad Sci USA, 95 (5): 2653-8. [PMID:9482942]
70. Wu B, Murray JK, Andrews KL, Sham K, Long J, Aral J, Ligutti J, Amagasu S, Liu D, Zou A et al.. (2018) Discovery of Tarantula Venom-Derived NaV1.7-Inhibitory JzTx-V Peptide 5-Br-Trp24 Analogue AM-6120 with Systemic Block of Histamine-Induced Pruritis. J Med Chem, 61 (21): 9500-9512. [PMID:30346167]
71. Wu FF, Takahashi MP, Pegoraro E, Angelini C, Colleselli P, Cannon SC, Hoffman EP. (2001) A new mutation in a family with cold-aggravated myotonia disrupts Na(+) channel inactivation. Neurology, 56 (7): 878-84. [PMID:11294924]
72. Zimmer T, Bollensdorff C, Haufe V, Birch-Hirschfeld E, Benndorf K. (2002) Mouse heart Na+ channels: primary structure and function of two isoforms and alternatively spliced variants. Am J Physiol Heart Circ Physiol, 282 (3): H1007-17. [PMID:11834499]













