Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Natural/Endogenous Ligands
- Rank order lists
- Agonists
- Antagonists
- Immunopharmacology Comments
- Immuno Cell Type Associations
- Transduction Mechanisms
- Tissue Distribution
- Expression Datasets
- Functional Assays
- Physiological Functions
- Physiological Consequences of Altering Gene Expression
- Phenotypes, Alleles and Disease Models
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 395 | 11q12.2 | PTGDR2 | prostaglandin D2 receptor 2 | 27 |
| Mouse | 7 | 382 | 19 A | Ptgdr2 | prostaglandin D2 receptor 2 | 1,15 |
| Rat | 7 | 403 | 1q43 | Ptgdr2 | prostaglandin D2 receptor 2 | 33 |
Previous and Unofficial Names  |
|
| CRTH2 [31] | CD294 | G protein-coupled receptor 44 | prostaglandin D2 receptor 2 | PGD2 receptor | Gpr44 | |
Database Links  |
|
| Specialist databases | |
| GPCRdb | pd2r2_human (Hs), pd2r2_mouse (Mm), pd2r2_rat (Rn) |
| Other databases | |
| Alphafold | Q9Y5Y4 (Hs), Q9Z2J6 (Mm), Q6XKD3 (Rn) |
| ChEMBL Target | CHEMBL5071 (Hs), CHEMBL2291 (Mm), CHEMBL1075112 (Rn) |
| Ensembl Gene | ENSG00000183134 (Hs), ENSMUSG00000034117 (Mm), ENSRNOG00000036631 (Rn) |
| Entrez Gene | 11251 (Hs), 14764 (Mm), 309212 (Rn) |
| Human Protein Atlas | ENSG00000183134 (Hs) |
| KEGG Gene | hsa:11251 (Hs), mmu:14764 (Mm), rno:309212 (Rn) |
| OMIM | 604837 (Hs) |
| Pharos | Q9Y5Y4 (Hs) |
| RefSeq Nucleotide | NM_004778 (Hs), NM_009962 (Mm), NM_001012070 (Rn) |
| RefSeq Protein | NP_004769 (Hs), NP_034092 (Mm), NP_001012070 (Rn) |
| UniProtKB | Q9Y5Y4 (Hs), Q9Z2J6 (Mm), Q6XKD3 (Rn) |
| Wikipedia | PTGDR2 (Hs) |
Selected 3D Structures  |
|||||||||||||
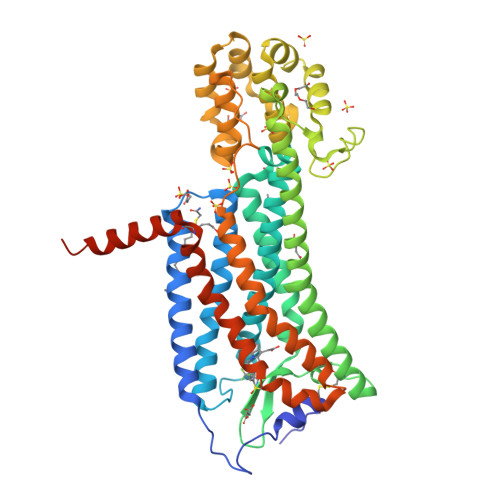
|
|
||||||||||||

|
|
||||||||||||
Natural/Endogenous Ligands  |
| PGD3 |
| PGD2 |
| PGE2 |
| PGF2α |
| PGI2 |
| PGJ2 |
| Comments: 11-Dehydro-thromboxane B2, a breakdown product of thromboxane A2 is an additional endogenous agonist of this receptor |
| Potency order of endogenous ligands |
| PGD2 >> PGF2α, PGE2 > PGI2, thromboxane A2 |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13,14-dihydro-15-oxo-PGD2 and 15R-15-methyl-PGD2 are selective DP2 agonists [15,25]. The COX-1/2 inhibitor indomethacin [16] and the thromboxane metabolite 11-dehydro TXB2 [6] are low-potency DP2 agonists. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The TP receptor antagonist ramatroban also blocks the DP2 receptor [35]. For a review on the use of DP2 antagonists to treat allergic inflammation see [22]. OC000459 antagonises [3H]PGD2 binding to native DP2 receptors in human Th2 lymphocytes with a Ki of 4nM [28]. ACT-453859 (structure not disclosed) is a clinical candidate DP2 receptor antagonist for allergic inflammation, predominantly for asthma [13,19,34]. AZD1981 is a selective DP2 receptor antagonist trialled for the treatment of allergic asthma [3]. DP2 antagonist TM30089 suppresses PGD2-dependent human ILC2 cell migration and cytokine production [43]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| The DP2 receptor (a.k.a. CRTH2, chemoattractant receptor-homologous molecule expressed on T-helper 2 cells) is expressed exclusively by a range of human immune cells including Th2 cells, basophils, eosinophils and innate lymphoid cells [17,26]. It mediates the proinflammatory effects of mast cell-derived prostaglandin D2 (PGD2) [26]. The DP2/PGD2 axis regulates the activation and chemotaxis of DP2 receptor +ve cells, and downstream pro-inflammatory events including stimulation of Th2 cytokine synthesis, local vasodilation and edema. Eosinophil morphology changes are promoted by PGD2-induced DP2 activation, as is enhanced migration from the bone marrow. Eosinophil degranulation and chemotactic responsiveness are also enhanced by this pathway. DP2-expressing type 2 innate lymphoid cells (ILC2) respond to and migrate toward PGD2 in vitro. Mice deficient in Ptgdr2 exhibited reduced ILC2 responses and inflammation in a murine model of helminth-induced pulmonary type 2 inflammation [41]. In human lung tissue specimens both DP1 and DP2 receptors are detected on alveolar macrophages, and activation of these two receptors aggravates airway neutrophilia in a mouse model [18]. Selective DP2 receptor antagonists are being actively sought as anti-inflammatory agents. AZD1981 has recently been trialled in a large multi-centred Phase 2b study (n=1,140 patients) for the novel treatment of allergic asthma [3], GB001/ADC3680B (Gossamer Bio, no structure disclosed) is also in Phase 2 for asthma [2], and fevipiprant has advanced to Phase 3 evaluation for asthma and other inflammatory airway conditions. Several more antagonists have demonstrated efficacy in animal models of inflammatory diseases. |
| Cell Type Associations | ||||||||
|
||||||||
|
||||||||
|
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gi/Go family | Adenylyl cyclase inhibition |
| References: 15,31 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
| Physiological Functions Comments | ||||||||
| DP2-mediated activation has been reported in canine granulocytes [32]. | ||||||||
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
1. Abe H, Takeshita T, Nagata K, Arita T, Endo Y, Fujita T, Takayama H, Kubo M, Sugamura K. (1999) Molecular cloning, chromosome mapping and characterization of the mouse CRTH2 gene, a putative member of the leukocyte chemoattractant receptor family. Gene, 227 (1): 71-7. [PMID:9931443]
2. Asano K, Sagara H, Ichinose M, Hirata M, Nakajima A, Ortega H, Tohda Y. (2020) A Phase 2a Study of DP2 Antagonist GB001 for Asthma. J Allergy Clin Immunol Pract, 8 (4): 1275-1283.e1. [PMID:31778823]
3. Bateman ED, O'Brien C, Rugman P, Luke S, Ivanov S, Uddin M. (2018) Efficacy and safety of the CRTh2 antagonist AZD1981 as add-on therapy to inhaled corticosteroids and long-acting β2-agonists in patients with atopic asthma. Drug Des Devel Ther, 12: 1093-1106. [PMID:29765200]
4. Brittan JE, King CD, Stearns BA. (2011) DP2 ANTAGONIST AND USES THEREOF. Patent number: WO2011085033. Assignee: PANMIRA PHARMACEUTICALS. Priority date: 06/01/2010. Publication date: 14/07/2011.
5. Burgess LE, Clark CT, Cook A, Corrette CP, Delise RK, Doherty GA, Hunt KW, Romoff T. (2009) 6-substituted phenoxychroman carboxylic acid derivatives. Patent number: WO2009158426A1. Assignee: Array Biopharma Inc.. Priority date: 25/06/2008. Publication date: 30/12/2009.
6. Böhm E, Sturm GJ, Weiglhofer I, Sandig H, Shichijo M, McNamee A, Pease JE, Kollroser M, Peskar BA, Heinemann A. (2004) 11-Dehydro-thromboxane B2, a stable thromboxane metabolite, is a full agonist of chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) in human eosinophils and basophils. J Biol Chem, 279 (9): 7663-70. [PMID:14668348]
7. Chevalier E, Stock J, Fisher T, Dupont M, Fric M, Fargeau H, Leport M, Soler S, Fabien S, Pruniaux MP et al.. (2005) Cutting edge: chemoattractant receptor-homologous molecule expressed on Th2 cells plays a restricting role on IL-5 production and eosinophil recruitment. J Immunol, 175 (4): 2056-60. [PMID:16081770]
8. Crosignani S, Jorand-Lebrun C, Campbell G, Prêtre A, Grippi-Vallotton T, Quattropani A, Bouscary-Desforges G, Bombrun A, Missotten M, Humbert Y et al.. (2011) Discovery of a Novel Series of CRTH2 (DP2) Receptor Antagonists Devoid of Carboxylic Acids. ACS Med Chem Lett, 2 (12): 938-42. [PMID:24900284]
9. Fretz H, Valdenaire A, Pothier J, Hilpert K, Gnerre C, Peter O, Leroy X, Riederer MA. (2013) Identification of 2-(2-(1-naphthoyl)-8-fluoro-3,4-dihydro-1H-pyrido[4,3-b]indol-5(2H)-yl)acetic acid (setipiprant/ACT-129968), a potent, selective, and orally bioavailable chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) antagonist. J Med Chem, 56 (12): 4899-911. [PMID:23721423]
10. Gazi L, Gyles S, Rose J, Lees S, Allan C, Xue L, Jassal R, Speight G, Gamble V, Pettipher R. (2005) Delta12-prostaglandin D2 is a potent and selective CRTH2 receptor agonist and causes activation of human eosinophils and Th2 lymphocytes. Prostaglandins Other Lipid Mediat, 75 (1-4): 153-67. [PMID:15789622]
11. Gervais FG, Cruz RP, Chateauneuf A, Gale S, Sawyer N, Nantel F, Metters KM, O'neill GP. (2001) Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol, 108 (6): 982-8. [PMID:11742277]
12. Gervais FG, Morello JP, Beaulieu C, Sawyer N, Denis D, Greig G, Malebranche AD, O'Neill GP. (2005) Identification of a potent and selective synthetic agonist at the CRTH2 receptor. Mol Pharmacol, 67 (6): 1834-9. [PMID:15755909]
13. Géhin M, Strasser DS, Zisowsky J, Farine H, Groenen PM, Dingemanse J, Sidharta PN. (2015) A novel CRTH2 antagonist: Single- and multiple-dose tolerability, pharmacokinetics, and pharmacodynamics of ACT-453859 in healthy subjects. J Clin Pharmacol, 55 (7): 787-97. [PMID:25655470]
14. Hata AN, Lybrand TP, Breyer RM. (2005) Identification of determinants of ligand binding affinity and selectivity in the prostaglandin D2 receptor CRTH2. J Biol Chem, 280: 32442-32451. [PMID:16030019]
15. Hata AN, Zent R, Breyer MD, Breyer RM. (2003) Expression and molecular pharmacology of the mouse CRTH2 receptor. J Pharmacol Exp Ther, 306 (2): 463-70. [PMID:12721327]
16. Hirai H, Tanaka K, Takano S, Ichimasa M, Nakamura M, Nagata K. (2002) Cutting edge: agonistic effect of indomethacin on a prostaglandin D2 receptor, CRTH2. J Immunol, 168 (3): 981-5. [PMID:11801628]
17. Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, Ichimasa M, Sugamura K, Nakamura M, Takano S et al.. (2001) Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med, 193 (2): 255-61. [PMID:11208866]
18. Jandl K, Stacher E, Bálint Z, Sturm EM, Maric J, Peinhaupt M, Luschnig P, Aringer I, Fauland A, Konya V et al.. (2016) Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung. J Allergy Clin Immunol, 137 (3): 833-43. [PMID:26792210]
19. Krause A, Zisowsky J, Strasser DS, Gehin M, Sidharta PN, Groenen PMA, Dingemanse J. (2016) Pharmacokinetic/Pharmacodynamic Modelling of Receptor Internalization with CRTH2 Antagonists to Optimize Dose Selection. Clin Pharmacokinet, 55 (7): 813-821. [PMID:26692193]
20. Liu J, Li AR, Wang Y, Johnson MG, Su Y, Shen W, Wang X, Lively S, Brown M, Lai S et al.. (2011) Discovery of AMG 853, a CRTH2 and DP Dual Antagonist. ACS Med Chem Lett, 2 (5): 326-30. [PMID:24900313]
21. Luker T, Bonnert R, Brough S, Cook AR, Dickinson MR, Dougall I, Logan C, Mohammed RT, Paine S, Sanganee HJ et al.. (2011) Substituted indole-1-acetic acids as potent and selective CRTh2 antagonists-discovery of AZD1981. Bioorg Med Chem Lett, 21 (21): 6288-92. [PMID:21944852]
22. Ly TW, Bacon KB. (2005) Small-molecule CRTH2 antagonists for the treatment of allergic inflammation: an overview. Expert Opin Investig Drugs, 14 (7): 769-73. [PMID:16022566]
23. Mathiesen JM, Christopoulos A, Ulven T, Royer JF, Campillo M, Heinemann A, Pardo L, Kostenis E. (2006) On the mechanism of interaction of potent surmountable and insurmountable antagonists with the prostaglandin D2 receptor CRTH2. Mol Pharmacol, 69 (4): 1441-53. [PMID:16418339]
24. Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. (2011) Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol, 12 (11): 1055-62. [PMID:21909091]
25. Monneret G, Cossette C, Gravel S, Rokach J, Powell WS. (2003) 15R-methyl-prostaglandin D2 is a potent and selective CRTH2/DP2 receptor agonist in human eosinophils. J Pharmacol Exp Ther, 304 (1): 349-55. [PMID:12490611]
26. Nagata K, Hirai H, Tanaka K, Ogawa K, Aso T, Sugamura K, Nakamura M, Takano S. (1999) CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s). FEBS Lett, 459 (2): 195-9. [PMID:10518017]
27. Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, Abe H, Tada K, Nakamura M, Sugamura K et al.. (1999) Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol, 162 (3): 1278-86. [PMID:9973380]
28. Pettipher R, Vinall SL, Xue L, Speight G, Townsend ER, Gazi L, Whelan CJ, Armer RE, Payton MA, Hunter MG. (2012) Pharmacologic profile of OC000459, a potent, selective, and orally active D prostanoid receptor 2 antagonist that inhibits mast cell-dependent activation of T helper 2 lymphocytes and eosinophils. J Pharmacol Exp Ther, 340 (2): 473-82. [PMID:22106101]
29. Royer JF, Schratl P, Lorenz S, Kostenis E, Ulven T, Schuligoi R, Peskar BA, Heinemann A. (2007) A novel antagonist of CRTH2 blocks eosinophil release from bone marrow, chemotaxis and respiratory burst. Allergy, 62 (12): 1401-9. [PMID:17714552]
30. Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, Yokozeki H, Hirai H, Nagata K, Hara T et al.. (2006) Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol, 177 (4): 2621-9. [PMID:16888024]
31. Sawyer N, Cauchon E, Chateauneuf A, Cruz RP, Nicholson DW, Metters KM, O'Neill GP, Gervais FG. (2002) Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br J Pharmacol, 137 (8): 1163-72. [PMID:12466225]
32. Schmidt JA, Bell FM, Akam E, Marshall C, Dainty IA, Heinemann A, Dougall IG, Bonnert RV, Sargent CA. (2013) Biochemical and pharmacological characterization of AZD1981, an orally available selective DP2 antagonist in clinical development for asthma. Br J Pharmacol, 168 (7): 1626-38. [PMID:23146091]
33. Shichijo M, Sugimoto H, Nagao K, Inbe H, Encinas JA, Takeshita K, Bacon KB, Gantner F. (2003) Chemoattractant receptor-homologous molecule expressed on Th2 cells activation in vivo increases blood leukocyte counts and its blockade abrogates 13,14-dihydro-15-keto-prostaglandin D2-induced eosinophilia in rats. J Pharmacol Exp Ther, 307 (2): 518-25. [PMID:12975488]
34. Srinivas NR. (2015) First-in-man study of ACT-453859, a potent CRTH2 antagonist--Is the metabolite formation influenced by a polymorphic enzyme?. J Clin Pharmacol, 55 (12): 1432. [PMID:26761218]
35. Sugimoto H, Shichijo M, Iino T, Manabe Y, Watanabe A, Shimazaki M, Gantner F, Bacon KB. (2003) An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J Pharmacol Exp Ther, 305 (1): 347-52. [PMID:12649388]
36. Sugimoto H, Shichijo M, Okano M, Bacon KB. (2005) CRTH2-specific binding characteristics of [3H]ramatroban and its effects on PGD2-, 15-deoxy-Delta12, 14-PGJ2- and indomethacin-induced agonist responses. Eur J Pharmacol, 524 (1-3): 30-7. [PMID:16256979]
37. Sykes D, Bradley M, Riddy D, Willard L, Powell-Herlaar E, Sandham D, Watson S, Bauer C, Dubois G, Charlton S. (2014) QAW039, a slowly dissociating CRTh2 antagonist with potential for improved clinical efficacy. Eur Respir J, 44 (Supl 58): 4074.
38. Sykes DA, Bradley ME, Riddy DM, Willard E, Reilly J, Miah A, Bauer C, Watson SJ, Sandham DA, Dubois G et al.. (2016) Fevipiprant (QAW039), a Slowly Dissociating CRTh2 Antagonist with the Potential for Improved Clinical Efficacy. Mol Pharmacol, 89 (5): 593-605. [PMID:26916831]
39. Ulven T, Kostenis E. (2005) Minor structural modifications convert the dual TP/CRTH2 antagonist ramatroban into a highly selective and potent CRTH2 antagonist. J Med Chem, 48 (4): 897-900. [PMID:15715457]
40. Wang L, Yao D, Deepak RNVK, Liu H, Xiao Q, Fan H, Gong W, Wei Z, Zhang C. (2018) Structures of the Human PGD2 Receptor CRTH2 Reveal Novel Mechanisms for Ligand Recognition. Mol Cell, 72 (1): 48-59.e4. [PMID:30220562]
41. Wojno ED, Monticelli LA, Tran SV, Alenghat T, Osborne LC, Thome JJ, Willis C, Budelsky A, Farber DL, Artis D. (2015) The prostaglandin D₂ receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol, 8 (6): 1313-23. [PMID:25850654]
42. Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, Pettipher R. (2005) Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol, 175 (10): 6531-6. [PMID:16272307]
43. Xue L, Salimi M, Panse I, Mjösberg JM, McKenzie AN, Spits H, Klenerman P, Ogg G. (2014) Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol, 133 (4): 1184-94. [PMID:24388011]

 Target has curated data in GtoImmuPdb
Target has curated data in GtoImmuPdb