|
Synonyms: AVL 292 | AVL-292 | cc-292
Compound class:
Synthetic organic
Comment: Spebrutinib acts as an inhibitor of Bruton's tyrosine kinase (BTK).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
IUPAC Name  |
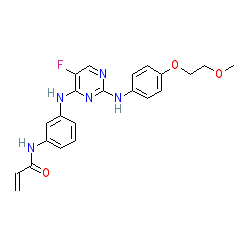
| N-[3-[[5-fluoro-2-[4-(2-methoxyethoxy)anilino]pyrimidin-4-yl]amino]phenyl]prop-2-enamide |
International Nonproprietary Names  |
|
| INN number | INN |
| 10004 | spebrutinib |
Synonyms  |
| AVL 292 | AVL-292 | cc-292 |
Database Links  |
|
| CAS Registry No. | 1202757-89-8 |
| ChEMBL Ligand | CHEMBL3301625 |
| GtoPdb PubChem SID | 223366168 |
| PubChem CID | 59174488 |
| Search Google for chemical match using the InChIKey | KXBDTLQSDKGAEB-UHFFFAOYSA-N |
| Search Google for chemicals with the same backbone | KXBDTLQSDKGAEB |
| Search PubMed clinical trials | spebrutinib |
| Search PubMed titles | spebrutinib |
| Search PubMed titles/abstracts | spebrutinib |
| UniChem Compound Search for chemical match using the InChIKey | KXBDTLQSDKGAEB-UHFFFAOYSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | KXBDTLQSDKGAEB-UHFFFAOYSA-N |