|
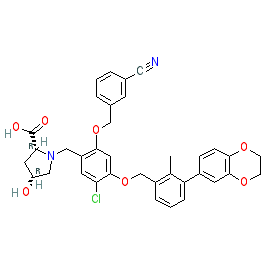
Synonyms: compound 2c [PMID: 28613862] | Example 1166 [WO2015160641A2]
Compound class:
Synthetic organic
Comment: BMS-1166 is a small molecule that disrupts the PD-1/PD-L1 immune checkpoint interaction. It is the most potent of the [3-(2,3-Dihydro-1,4-benzodioxin-6-yl)-2-methylphenyl]methanol derivatives described in [2], and it is claimed as Example 1166 in Bristol-Myers Squibb's patent WO2015160641A2 [1]. NMR analysis suggests that BMS-1166 promotes the formation of dimeric PD-L1 in solution.
|
|
|||||||||||||||||||||||||||||||||||
For advanced searching click here to open chemical structure editor
Similar Ligands  |
|||||
|
|||||