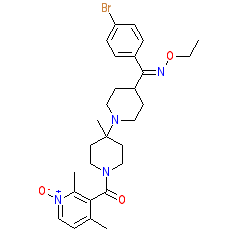
|
Synonyms: SCH 351125 | SCH-351125 | SCH-C
Compound class:
Synthetic organic
Comment: Ancriviroc (SCH351125) is an orally available, small molecule antagonist of the C-C chemokine receptor type 5 (CCR5) expressed on T cells [4]. The compound was originally investigated as an anti-HIV therapeutic [4]. SCH351125-induced CCR5 blockade was investigated for action in treating rheumatoid arthritis, but with discouraging clinical trial results [6]. Other CCR5 antagonists (AZD5672 and maraviroc) have also proven ineffective in RA clinical trials [5].
There is some ambiguity in the literature and online databases as to the exact structure of ancriviroc. The structure shown here matches that in the PubChem entry linked to above, and as shown in [4], while other common representations of ancriviroc match CID 5479787. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
For advanced searching click here to open chemical structure editor
Similar Ligands  |
|||||
|
|||||