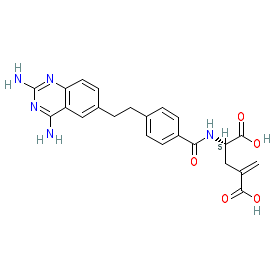
|
Synonyms: Formula (10) [WO2012078708A1]
Compound class:
Synthetic organic
Comment: CH-4051 is an orally available methotrexate (MTX) analogue that is designed to resist metabolic degradation [4-5]. This strategy was employed to improve the safety and tolerability profile compared to MTX, since a significant proportion of the toxicity profile of MTX can be attributed to its polyglutamylated and hydroxylated metabolites. Like MTX, CH-4051 has anti-inflammatory, autoimmune and anti-tumour properties and is a potent inhibitor of dihydrofolate reductase, an enzyme required for cell proliferation. The racemic mixture of CH-4051, known as CH-1405 (represented by the non-chiral structure in PubChem CID 9846537) [3], completed Phase 2 development for rheumatoid arthritis, and exhibited comparable efficacy to MTX [1-2]. Structurally, CH-4051 is the more active L-4'-methylene-glutamic acid containing diastereomer (or S-enantiomer) of CH-1405 [5].
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Bajpai M. (2010)
CH-1504, a metabolically inert antifolate for the potential treatment of rheumatoid arthritis. IDrugs, 13 (8): 559-67. [PMID:20721827] |
|
2. Keystone EC, Shirinsky VS, Simon LS, Pedder S, Hewitt LA, CH-1504 Study Group. (2011)
Efficacy and safety of CH-1504, a metabolically stable antifolate, in patients with active rheumatoid arthritis: results of a phase II multicenter randomized study. J Rheumatol, 38 (9): 1875-83. [PMID:21724705] |
|
3. McGuire JJ, Haile WH. (2009)
Metabolism-blocked antifolates as potential anti-rheumatoid arthritis agents: 4-amino-4-deoxy-5,8,10-trideazapteroyl-d,l-4'-methyleneglutamic acid (CH-1504) and its analogs. Biochem Pharmacol, 77 (7): 1161-72. [PMID:19174154] |
|
4. Pimplaskar HK, Lebedev M, Horvath K. (2009)
Crystalline salt forms of antifolate compounds and methods of manufacturing thereof. Patent number: WO2009126639A1. Assignee: Chelsea Therapeutics, Inc.. Priority date: 07/04/2008. Publication date: 15/10/2009. |
|
5. Roberts MJ, Rowse G. (2012)
Combination comprising methotrexate and an antifolate compound. Patent number: WO2012078708A1. Assignee: Chelsea Therapeutics, Inc.. Priority date: 07/12/2010. Publication date: 14/06/2012. |