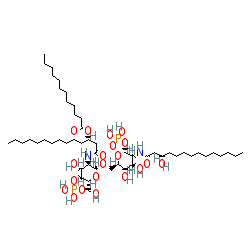
|
Synonyms: OM 174 | OM-174
Compound class:
Synthetic organic
Comment: Defoslimod is an analogue of lipopolysaccharide endotoxin-derived lipid A obtained from E. coli [1]. It was developed as an immunotherapeutic agent for the treatment of cancer. Defoslimod acts as an agonist of Toll-like receptors 2 and 4 (TLR2 and TLR4) [2].
We show the structure with chemical formula provided in the INN record for the compound (C52H100N2O20P2). PubChem CID 56841121, which claims to represent defoslimod has an extra carbon atom and two associated hydrogens (C53H102N2O20P2). But note also that the structure reported in [1] only has 51 carbon atoms. |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Brandenburg K, Lindner B, Schromm A, Koch MH, Bauer J, Merkli A, Zbaeren C, Davies JG, Seydel U. (2000)
Physicochemical characteristics of triacyl lipid A partial structure OM-174 in relation to biological activity. Eur J Biochem, 267 (11): 3370-7. [PMID:10824125] |
|
2. Garay RP, Viens P, Bauer J, Normier G, Bardou M, Jeannin JF, Chiavaroli C. (2007)
Cancer relapse under chemotherapy: why TLR2/4 receptor agonists can help. Eur J Pharmacol, 563 (1-3): 1-17. [PMID:17383632] |
|
3. Isambert N, Fumoleau P, Paul C, Ferrand C, Zanetta S, Bauer J, Ragot K, Lizard G, Jeannin JF, Bardou M. (2013)
Phase I study of OM-174, a lipid A analogue, with assessment of immunological response, in patients with refractory solid tumors. BMC Cancer, 13: 172. [PMID:23547558] |
|
4. Meraldi V, Audran R, Romero JF, Brossard V, Bauer J, López JA, Corradin G. (2003)
OM-174, a new adjuvant with a potential for human use, induces a protective response when administered with the synthetic C-terminal fragment 242-310 from the circumsporozoite protein of Plasmodium berghei. Vaccine, 21 (19-20): 2485-91. [PMID:12744882] |
|
5. Onier N, Hilpert S, Arnould L, Saint-Giorgio V, Davies JG, Jeannin JF, Jeannin JF. (1999)
Cure of colon cancer metastasis in rats with the new lipid A OM 174. Apoptosis of tumor cells and immunization of rats. Clin Exp Metastasis, 17 (4): 299-306. [PMID:10545016] |