|
Synonyms: AZD-5069
Compound class:
Synthetic organic
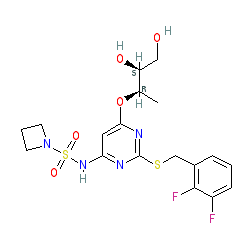
Comment: AZD5069 is a novel, selective antagonist of the chemokine receptor, CXCR2. The structure shown here was drawn from that in [6], which specifies stereochemistry. This structure is identical to Example 47 in patent US7838675 [1]. PubChem records the structure with no specified stereochemistry (CID 59558839).
Small-molecule antagonists of CXCR2 are being developed for their potential to treat inflammatory conditions. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Cheshire DR, Cox RJ, Meghani P, Stonehouse JP. (2010)
Pyrimidine sulphonamide derivatives as chemokine receptor modulators. Patent number: US7838675 B2. Assignee: Astrazeneca Ab.. Priority date: 28/08/2004. Publication date: 23/11/2010. |
|
2. De Soyza A, Pavord I, Elborn JS, Smith D, Wray H, Puu M, Larsson B, Stockley R. (2015)
A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur Respir J, 46 (4): 1021-32. [PMID:26341987] |
|
3. Jurcevic S, Humfrey C, Uddin M, Warrington S, Larsson B, Keen C. (2015)
The effect of a selective CXCR2 antagonist (AZD5069) on human blood neutrophil count and innate immune functions. Br J Clin Pharmacol, 80 (6): 1324-36. [PMID:26182832] |
|
4. Kirsten AM, Förster K, Radeczky E, Linnhoff A, Balint B, Watz H, Wray H, Salkeld L, Cullberg M, Larsson B. (2015)
The safety and tolerability of oral AZD5069, a selective CXCR2 antagonist, in patients with moderate-to-severe COPD. Pulm Pharmacol Ther, 31: 36-41. [PMID:25681277] |
|
5. Mårdh CK, Root J, Uddin M, Stenvall K, Malmgren A, Karabelas K, Thomas M. (2017)
Targets of Neutrophil Influx and Weaponry: Therapeutic Opportunities for Chronic Obstructive Airway Disease. J Immunol Res, 2017: 5273201. [PMID:28596972] |
|
6. Nicholls DJ, Wiley K, Dainty I, MacIntosh F, Phillips C, Gaw A, Mårdh CK. (2015)
Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist. J Pharmacol Exp Ther, 353 (2): 340-50. [PMID:25736418] |
|
7. O'Byrne PM, Metev H, Puu M, Richter K, Keen C, Uddin M, Larsson B, Cullberg M, Nair P. (2016)
Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med, 4 (10): 797-806. [PMID:27574788] |
|
8. Pedersen F, Waschki B, Marwitz S, Goldmann T, Kirsten A, Malmgren A, Rabe KF, Uddin M, Watz H. (2018)
Neutrophil extracellular trap formation is regulated by CXCR2 in COPD neutrophils. Eur Respir J, 51 (4). [PMID:29449427] |
|
9. Uddin M, Betts C, Robinson I, Malmgren A, Humfrey C. (2017)
The chemokine CXCR2 antagonist (AZD5069) preserves neutrophil-mediated host immunity in non-human primates. Haematologica, 102 (2): e65-e68. [PMID:27742769] |
|
10. Watz H, Uddin M, Pedersen F, Kirsten A, Goldmann T, Stellmacher F, Groth E, Larsson B, Böttcher G, Malmgren A et al.. (2017)
Effects of the CXCR2 antagonist AZD5069 on lung neutrophil recruitment in asthma. Pulm Pharmacol Ther, 45: 121-123. [PMID:28549850] |