|
Synonyms: Entocort® | Eohilia® (budesonide oral suspension) | Jorveza® | Pulmicort® | S-1320 | Uceris®
budesonide is an approved drug (FDA (1997), EMA (2018))
Compound class:
Synthetic organic
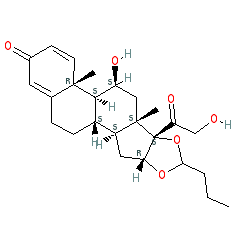
Comment: Budesonide is a glucocorticoid steroid. The INN document specifies that the approved drug consists of a racemic mixture of two epimers, based on the stereocentre of the carbon where the butyl chain meets the rest of the molecule. Our structure does not specify strereochemistry at this point and represents the mixture. The two epimers are represented by CID 40000 and CID 63006.
COVID-19: Results from a Phase 2 clinical trial have shown that a short duration administration of budesonide in the early phase of SARS-CoV-2 infection reduces both the likelihood of needing urgent medical care and time to recovery [5]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: budesonide |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Habal FM, Huang VW. (2012)
Review article: a decision-making algorithm for the management of pregnancy in the inflammatory bowel disease patient. Aliment Pharmacol Ther, 35 (5): 501-15. [PMID:22221203] |
|
2. Hemmerling M, Nilsson S, Edman K, Eirefelt S, Russell W, Hendrickx R, Johnsson E, Kärrman Mårdh C, Berger M, Rehwinkel H et al.. (2017)
Selective Nonsteroidal Glucocorticoid Receptor Modulators for the Inhaled Treatment of Pulmonary Diseases. J Med Chem, 60 (20): 8591-8605. [PMID:28937774] |
|
3. Lichtenstein GR, Hanauer SB, Sandborn WJ, Practice Parameters Committee of American College of Gastroenterology. (2009)
Management of Crohn's disease in adults. Am J Gastroenterol, 104 (2): 465-83; quiz 464, 484. [PMID:19174807] |
|
4. Millan DS, Ballard SA, Chunn S, Dybowski JA, Fulton CK, Glossop PA, Guillabert E, Hewson CA, Jones RM, Lamb DJ et al.. (2011)
Design and synthesis of long acting inhaled corticosteroids for the treatment of asthma. Bioorg Med Chem Lett, 21 (19): 5826-30. [PMID:21880489] |
|
5. Ramakrishnan S, Nicolau Jr DV, Langford B, Mahdi M, Jeffers H, Mwasuku C, Krassowska K, Fox R, Binnian I, Glover V et al.. (2021)
Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med, 9 (7): 763-772. [PMID:33844996] |