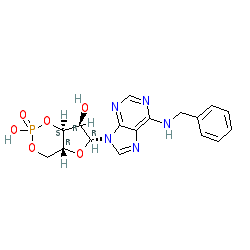
|
Synonyms: N6-benzyl cyclic AMP
Compound class:
Synthetic organic
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG et al.. (2003)
cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem, 278 (37): 35394-402. [PMID:12819211] |