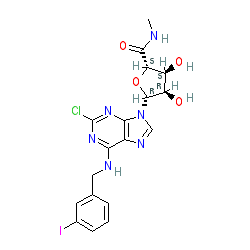
|
Synonyms: 2Cl-IB-MECA
Compound class:
Synthetic organic
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Brambilla R, Cattabeni F, Ceruti S, Barbieri D, Franceschi C, Kim YC, Jacobson KA, Klotz KN, Lohse MJ, Abbracchio MP. (2000)
Activation of the A3 adenosine receptor affects cell cycle progression and cell growth. Naunyn Schmiedebergs Arch Pharmacol, 361 (3): 225-34. [PMID:10731034] |
|
2. Jacobson KA, Gao ZG. (2006)
Adenosine receptors as therapeutic targets. Nat Rev Drug Discov, 5 (3): 247-64. [PMID:16518376] |
|
3. Jacobson KA, Park KS, Jiang JL, Kim YC, Olah ME, Stiles GL, Ji XD. (1997)
Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology, 36 (9): 1157-65. [PMID:9364471] |
|
4. Kim HO, Ji XD, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. (1994)
2-Substitution of N6-benzyladenosine-5'-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem, 37 (21): 3614-21. [PMID:7932588] |
|
5. Liang BT, Urso R, Sambraski E, Jacobson KA. (2010)
. In A3 Adenosine Receptors from Cell Biology to Pharmacology and Therapeutics Edited by Borea PA (Springer) . [ISBN:9789048131440] |