|
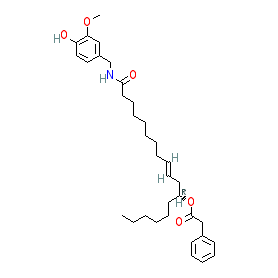
Synonyms: 12-Phenylacetyl-ricinoleoyl-vanillamide [1] | compound 1e [PMID: 15356216] | IDN5890 | PhAR
Compound class:
Synthetic organic
Comment: Phenylacetylrinvanil is a 'capsaicinoid' type compound that is a potent agonist/activator at the transient receptor potential vanilloid type 1 (TRPV1) channel [2]. Structurally it is a hybrid molecule that combines a vanillamide TRPV1 agonist with a non-polyunsaturated fatty acid-derived cannabinoid CB2 receptor-selective ligand. Phenylacetylrinvanil has demonstrated some antineoplastic activity against leukaemia cells in vitro and in vivo [3] and against cervical cancer tumour cell lines [4].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Appendino G, Cascio MG, Bacchiega S, Moriello AS, Minassi A, Thomas A, Ross R, Pertwee R, De Petrocellis L, Di Marzo V. (2006)
First "hybrid" ligands of vanilloid TRPV1 and cannabinoid CB2 receptors and non-polyunsaturated fatty acid-derived CB2-selective ligands. FEBS Lett, 580 (2): 568-74. [PMID:16406364] |
|
2. Appendino G, De Petrocellis L, Trevisani M, Minassi A, Daddario N, Moriello AS, Gazzieri D, Ligresti A, Campi B, Fontana G et al.. (2005)
Development of the first ultra-potent "capsaicinoid" agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J Pharmacol Exp Ther, 312 (2): 561-70. [PMID:15356216] |
|
3. Luviano A, Aguiñiga-Sánchez I, Demare P, Tiburcio R, Ledesma-Martínez E, Santiago-Osorio E, Regla I. (2014)
Antineoplastic activity of rinvanil and phenylacetylrinvanil in leukaemia cell lines. Oncol Lett, 7 (5): 1651-1656. [PMID:24765194] |
|
4. Sánchez-Sánchez L, Alvarado-Sansininea JJ, Escobar ML, López-Muñoz H, Hernández-Vázquez JM, Monsalvo-Montiel I, Demare P, Regla I, Weiss-Steider B. (2015)
Evaluation of the antitumour activity of Rinvanil and Phenylacetylrinvanil on the cervical cancer tumour cell lines HeLa, CaSKi and ViBo. Eur J Pharmacol, 758: 129-36. [PMID:25864613] |