|
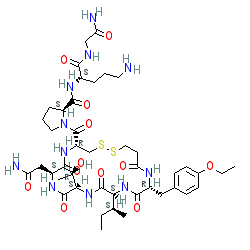
Synonyms: Antocin® | d[D-Tyr(Et)2,Thr4,Orn8]vasotocin | d[D-Tyr(Et)2,Thr4]OVT | ORF22164 | RWJ 22164 | Tractocile®
atosiban is an approved drug (EMA (2000))
Compound class:
Peptide or derivative
Comment: A synthetic analogue of oxytocin. There is some ambiguity over the exact stereochemistry of atosiban. Another representation is CID 68613.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
| References |
|
1. Akerlund M, Bossmar T, Brouard R, Kostrzewska A, Laudanski T, Lemancewicz A, Serradeil-Le Gal C, Steinwall M. (1999)
Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol, 106 (10): 1047-53. [PMID:10519430] |
|
2. Cirillo R, Gillio Tos E, Schwarz MK, Quattropani A, Scheer A, Missotten M, Dorbais J, Nichols A, Borrelli F, Giachetti C et al.. (2003)
Pharmacology of (2S,4Z)-N-[(2S)-2-hydroxy-2-phenylethyl]-4-(methoxyimino) -1-[(2'-methyl[1,1'-biphenyl]-4-yl)carbonyl]-2-pyrrolidinecarboxamide, a new potent and selective nonpeptide antagonist of the oxytocin receptor. J Pharmacol Exp Ther, 306 (1): 253-61. [PMID:12660315] |
|
3. Gimpl G, Fahrenholz F. (2001)
The oxytocin receptor system: structure, function, and regulation. Physiol Rev, 81 (2): 629-83. [PMID:11274341] |
|
4. Jasper JR, Harrell CM, O'Brien JA, Pettibone DJ. (1995)
Characterization of the human oxytocin receptor stably expressed in 293 human embryonic kidney cells. Life Sci, 57 (24): 2253-61. [PMID:7475979] |
|
5. Manning M, Cheng LL, Stoev S, Wo NC, Chan WY, Szeto HH, Durroux T, Mouillac B, Barberis C. (2005)
Design of peptide oxytocin antagonists with strikingly higher affinities and selectivities for the human oxytocin receptor than atosiban. J Pept Sci, 11 (10): 593-608. [PMID:15880385] |
|
6. Serradeil-Le Gal C, Valette G, Foulon L, Germain G, Advenier C, Naline E, Bardou M, Martinolle J-P, Pouzet B, Raufaste D, Garcia C, Double-Cazenave E, Pauly M, Pascal M, Barbier A, Scatton B, Maffrand J-P, Le Fur G. (2004)
SSR126768A (4-Chloro-3-[(3R)-(+)-5-chloro-1-(2,4-dimethoxybenzyl)-3-methyl-2oxo-2,3-dihydro-1H-indol-3-yl]-N-ethyl-N-(3-pyridylmethyl)-benzamidine, hydrochloride): a new selective and orally active oxytocin receptor antagonist for the prevention of preterm labor. J Pharmacol Exp Therap, 309: 414-424. [PMID:14722330] |