|
Compound class:
Synthetic organic
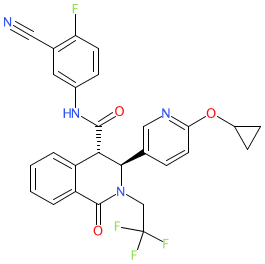
Comment: Compound 1a is from a series of novel tetrahydroisoquinoline derivatives, with antimalarial activity, claimed in patent WO2021204952A1 [1]. Antimalarial potency for this series is predominantly associated with the (+) enantiomer.
The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Guide to Malaria Pharmacology Comments |
| A patent search suggests that the back-up series for (+)-SJ733, being developed by the University of Kentucky in partnership with MMV, may be covered by patent WO2021204952 [1]. Compound 1a has similar in vitro asexual blood stage potency to (+)-SJ733 together with significantly improved metabolic stability. Potential Target/Mechanism Of Action: an increase of cytosolic sodium concentration following treatment with compound 1a is consistent with inhibition of Plasmodium non-SERCA-type Ca2+-transporting P-ATPase (PfATP4) [1]. |