|
Compound class:
Synthetic organic
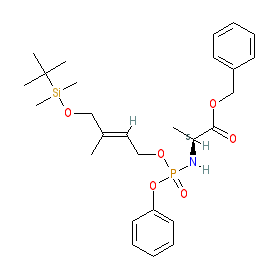
Comment: Vβ9/Vδ2 T-cells have been shown to be activated by small molecule phosphoantigens (PAgs). The aryloxy triester phosphoramidate prodrug ProPAgen 5b is a potent and specific activator of these cells as a potential immunotherapeutic agent [1]. The molecular mechanism of action is unkown but evidence supports PAgs binding the intracellular B30.2 domain of butyrophilin 3A1.
|
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Present since birth,1 Vγ9/Vδ2 T-cells represent the dominant subtype of human γδ T-cells in adult peripheral blood. They expand in response to various infections, including tuberculosis, leprosy, typhoid, malaria, and toxoplasmosis, and studies in primate models have suggested a role in immunity to Mycobacterium tuberculosis. Interestingly, they also exhibited an ability to target and lyse a diverse range of cancer cells in vitro. Such properties have made the Vγ9/Vδ2 subset a major focus in the therapeutic exploitation of γδ T-cells. |