|
Compound class:
Synthetic organic
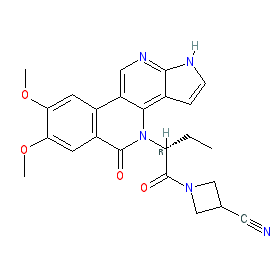
Comment: Inhibitor 20a represents a novel chemotype for Janus kinase inhibitors; benzo[ c]pyrrolo[2,3- h][1,6]naphthyridin-5-one (BPN) [1]. It was identified using sequential palladium catalysis.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Inhibitor 20a shows some selectivity for JAK1 compared to the other members of the Janus kinase family [1]. JAK inhibitors (e.g. tofacitinib and baricitinib) are used clinically as anti-inflammatory agents for the treatment of autoimmune diseases such as rheumatoid arthritis and ulcerative colitis. The search for more selective inhibitors with fewer adverse side-effects is continuing. Inhibitor 20a was designed and discovered with this potential advantage in mind. |