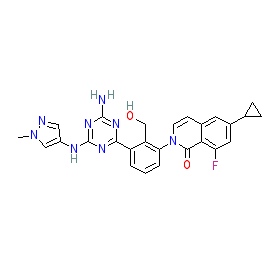
|
Synonyms: compound 4b [PMID: 30216722]
Compound class:
Synthetic organic
Comment: This compound is reported as a selective inhbitor of Bruton's tyrosine kinase (BTK) [2]. Compared to existing BTK inhibitors it has a novel aminotriazine scaffold. Inhibitor 4b has been advanced to preclinical studies for evaluation of its anti-inflammatory potential. This is one of the chemical structures claimed in Carna Bioscience's patent WO2015012149A1 [3]. The structure is identical to that for the INN sofnobrutinib.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Inhibitor 4b is a selective BTK inhibitor that is being evaluated for preclinical anti-inflammatory efficacy [2]. Based on in vivo tests in rodents it is being considered as a lead compound for the treatment of rheumatoid arthritis. BTK activity is implicated in a number of pathologies that are driven by B-cell or macrophage activation, such as B-cell malignancies, asthma, rheumatoid arthritis, and systemic lupus erythematosus. BTK inhibitors have already proven useful in the clinic (see ibrutinib and acalabrutinib). |