|
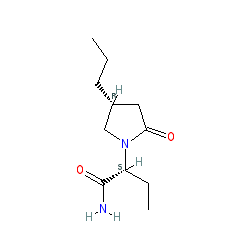
Synonyms: 2-(2-oxo-4-propylpyrrolidin-1-yl)butanamide | Briviact® | compound 83α [1] | UCB 34714 [3] | UCB-34714 | UCB34714
brivaracetam is an approved drug (EMA & FDA (2016))
Compound class:
Synthetic organic
Comment: Brivaracetam is a 4-n-propyl analogue of levetiracetam with anticonvulsant properties [3]. Like levetiracetam this drug inhibits synaptic vesicle protein 2A (SV2A), but with 10X higher binding affinity. Brivaracetam is brain penetrant and has a fast onset of action [2]. It can also inhibit sodium channels.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |