|
Synonyms: AZD-5069
Compound class:
Synthetic organic
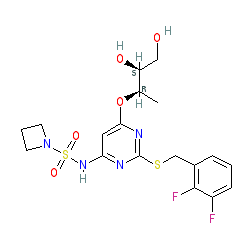
Comment: AZD5069 is a novel, selective antagonist of the chemokine receptor, CXCR2. The structure shown here was drawn from that in [6], which specifies stereochemistry. This structure is identical to Example 47 in patent US7838675 [1]. PubChem records the structure with no specified stereochemistry (CID 59558839).
Small-molecule antagonists of CXCR2 are being developed for their potential to treat inflammatory conditions. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| AZD5069 is being evaluated in Phase 2 clinical trials for inflammatory conditions and oncology indications. This compound has been extensively studied as an anti-inflammatory agent in chronic respiratory diseases, including severe asthma [7,10], COPD [4,8] and bronchiectasis [2]. Trials combining AZD5069 with the anti-PD-L1 immune checkpoint inhibitor durvalumab are underway in patients with metastatic pancreatic ductal adenocarcinoma (NCT02583477, Phase 1/2) and metastatic squamous cell carcinoma of the head and neck and other solid tumours (NCT02499328, Phase 1/2). For a list of all current AZD5069 trials, visit ClinicalTrials.gov. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02499328 | Study to Assess MEDI4736 With Either AZD9150 or AZD5069 in Advanced Solid Tumors & Relapsed Metastatic Squamous Cell Carcinoma of Head & Neck | Phase 1/Phase 2 Interventional | AstraZeneca | ||
| NCT02583477 | Phase Ib/II Study of MEDI4736 Evaluated in Different Combinations in Metastatic Pancreatic Ductal Carcinoma | Phase 1/Phase 2 Interventional | AstraZeneca | ||