|
Synonyms: BF2.649 | Ozawade® | tiprolisant [USAN] | Wakix®
pitolisant is an approved drug (EMA (2016), FDA (2019))
Compound class:
Synthetic organic
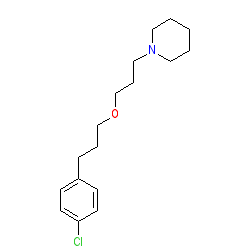
Comment: Pitolisant is a selective antagonist/inverse agonist of the histamine H3 receptor [7-8,11].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Pitolisant was initially granted orphan designation as a treatment for excessive daytime sleepiness and cataplexy in patients with narcolspey in the US and across Europe. In March 2016, pitolisant received EMA marketing authorisation for the treatment of narcolepsy [2]. Phase 3 trial results were reported in 2017 [10]. It was 2019 before the FDA approved this drug for the same indication as covered by the EMA marketing authorisation. In 2021, the EMA expanded authorisation to include treatment of obstructive sleep apnea. In Feb. 2024 the FDA granted orphan designation for the treatment of Prader-Willi syndrome. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Preclinical pharmacology of this compound is reported in [4-5], and its clinical potential is discussed in [1,3,9]. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) European Medicines Agency (EMA) |