|
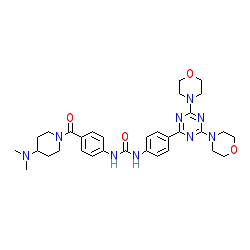
Synonyms: PF-05212384 | PKI 587 | PKI-587
Compound class:
Synthetic organic
Comment: Gedatolisib is an orally bioavailable small molecule inhibitor of phosphatidylinositol 3 kinase (PI3K) and mammalian target of rapamycin (mTOR) with potential antineoplastic activity [1-3].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Phase 2 clinical evaluations in AML and some solid tumour types were terminated. The AML study was abandoned as no objective response was observed at preliminary analysis and it was deemed futile to continue the trial. Many Phase 1 studies looking at the effect of gedatolisib in combination with other anti-cancer therapeutics, in advanced breast, lung, head and neck and pancreatic cancers, are underway. Click here to link to ClinicalTrials.gov's full list of gedatolisib trials. |