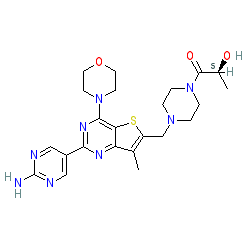
|
Synonyms: GDC-0980 | GDC0980 | RG-7422 | RG7422
Compound class:
Synthetic organic
Comment: Apitolisib is a dual PI3K/mTOR inhibitor. Its discovery is described in [5], where it is compound 2 (GDC-0980).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Apitolisib has been investigated in clinical trials for breast cancer, endometrial cancer, refractory solid tumours, non-Hodgkin's lymphoma and other advanced solid tumours such as prostate cancer and renal cell cancer. The most advanced stage of development was Phase 2. Click here to link to ClinicalTrials.gov's list of all apitolisib trials. |
Mechanism Of Action and Pharmacodynamic Effects  |
| PI3Ks and mTOR participate in related, but not redundant, signaling networks to transmit cellular growth and survival signals, which are hallmarks of tumor growth [1,3-4]. Due to their active site similarity, dual PI3K/mTOR inhibitors have been intensely investigated as antineoplastic agents [2,6]. |