|
Synonyms: AZD 9291 | AZD-9291 | AZD9291 | mereletinib (obsolete INN) | Tagrisso®
osimertinib is an approved drug (FDA (2015), EMA (2016))
Compound class:
Synthetic organic
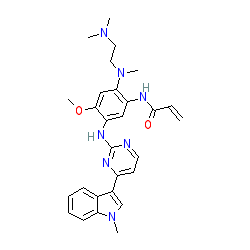
Comment: Osimertinib (AZD9291) is a third-generation, potent, selective and irreversible inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase bearing mutations which either sensitise the receptor, or cause drug resistance (eg the T790M mutation) [2]. The compound is less effective against the wild-type receptor.
This compound is included in AstaZeneca's Open Innovation Pharmacology Toolbox. There are reports of acquired resistance to osimertinib with tumours developing the C797S [6,9] and L718Q mutations [1] . Third-generation mutant EGFR inhibitors are being developed to circumvent dose-limiting WT EGFR-driven toxicities such as gastrointestinal toxicity and rash. The progress being made in developing advanced generation EGFR inhibitors is discussed by Yu and Riely (2013) [8] and Ou and Soo (2015) [4]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: osimertinib |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| A Phase 3 clinical trial (NCT02151981) is recruiting patients to compare AZD9291 vs. platinum-based doublet-chemotherapy in locally advanced or metastatic non-small cell lung cancer (Oct 2014). Click here to link to ClinicalTrials.gov's listing of AZD9291 trials. As a result of the FDA's accelerated approval program, osimertinib was approved in November 2015 for the treatment of patients with metastatic EGFRT790M mutation-positive NSCLC that has progressed on or after EGFR tyrosine kinase inhibitor therapy (and with the mutation confirmed using an FDA-approved test). This was converted to regular approval in March 2017. In April 2018 the FDA expanded osimertinib's approval to include use as a first-line treatment for metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations (again as detected by an FDA-approved test) [3]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04035486 | A Study of Osimertinib With or Without Chemotherapy as 1st Line Treatment in Patients With Mutated Epidermal Growth Factor Receptor Non-Small Cell Lung Cancer (FLAURA2) | Phase 3 Interventional | AstraZeneca | 5 | |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |