|
Synonyms: Flotros® | IP-631 | IP631 | Regurin® | Sanctura®
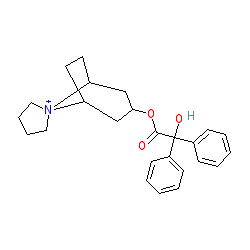
trospium is an approved drug (FDA (2004))
Compound class:
Synthetic organic
Comment: The marketed product contains trospium chloride (PubChem CID 107979). Note that the INN record for trospium chloride represents a chiral molecule, the closest PubChem match being AC1NR4SW. Our representation and those contained in our links show the non-isomeric molecule.
Trospium is a peripherally restricted muscarinic receptor antagonist. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Trospium chloride is a muscarinic antagonist used clinically as a urinary antispasmodic, indicated for the treatment of overactive bladder syndrome. An oral dual-drug fixed-dose combination of the M1/M4 receptor agonist xanomeline and trospium (known as KarXT™) is proposed as as novel schizophrenia therapy [1,3]. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Trospium chloride is a muscarinic antagonist [2]. The parasympatholytic action of trospium chloride, induces relaxation of the detrusor muscle in the wall of the bladder and reduces over all muscle tone in the bladder, thereby increasing volume and decreasing the symptoms of urge incontinence, urgency, and urinary frequency associated with overactive bladder syndrome. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03697252 | A Study to Assess Safety and Efficacy of KarXT in Adult Patients With Schizophrenia | Phase 2 Interventional | Karuna Therapeutics | ||
| NCT04659161 | A Study to Assess Efficacy and Safety of KarXT in Acutely Psychotic Hospitalized Adult Patients With Schizophrenia (EMERGENT-2) | Phase 3 Interventional | Karuna Therapeutics | ||
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com |