|
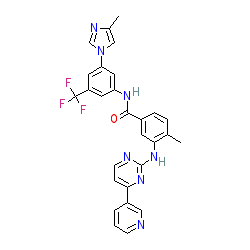
Synonyms: AMN 107 | AMN107 | Tasigna®
nilotinib is an approved drug (FDA & EMA (2007))
Compound class:
Synthetic organic
Comment: Nilotinib is a Type-2 kinase inhibitor and was first approved by the FDA in 2007.
Preclinical studies in rodent Parkinson's disease models suggest that nilotinib has some potential to promote autophagic degradation of α-synuclein [4-6,9], a brain protein whose accumulation and aggregation contributes to the formation of toxic insoluble fibrils which cause pathological changes such as neuronal loss in Parkinson's disease and other synucleinopathies. This mechanism has been evaluated in a pilot, proof-of-concept and safety clinical trial in a small number of patients with Parkinson's disease- and diffuse Lewy body disease-associated cognitive impairment (see NCT02281474; note that this trial is not placebo controlled or blinded). Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: nilotinib |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Used in the treatment of chronic myelogenous leukemia. Marketed formulations contain nilotinib hydrochloride monohydrate (PubChem CID 16757572). |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |