|
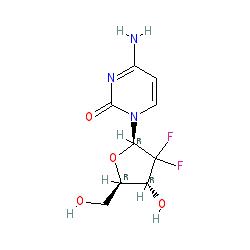
Synonyms: gemcitabine hydrochloride | Gemzar®
gemcitabine is an approved drug (FDA (1996))
Compound class:
Synthetic organic
Comment: Gemcitabine is a nucleoside analogue chemotherapeutic. Chemically, the hydrogen atoms on the 2' carbon of deoxycytidine are replaced by fluorine atoms.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Gemcitabine is a nucleoside analogue and its cytotoxicity is correlated with incorporation into genomic DNA and concomitant inhibition of DNA synthesis. Gemcitabine is metabolised by nucleoside kinases to the active diphosphate (dFdCDP) and triphosphate (dFdCTP) nucleosides: Gemcitabine diphosphate inhibits ribonucleotide reductase resulting in a reduction in the concentrations of deoxynucleotides, including dCTP in the cell. Concommitantly the gemcitabine triphosphate is preferentially incorporated into the DNA as a result of the diphosphate induced reduction in the dCTP level. In combination, these actions lead to inability of the cell to synthesise new DNA or repair damaged DNA and cells undergo apoptosis. Gemcitabine is also believed to inhibit topoisomerase I, since mouse leukemia cells with no topoisomerase I are resistant to gemcitabine induced-growth inhibition [3]. |