|
Synonyms: AT-527 | AT527 | RO-7496998 | RO7496998
Compound class:
Synthetic organic
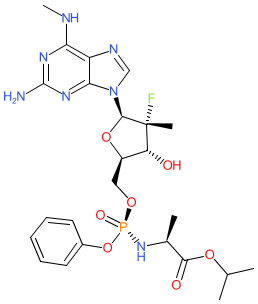
Comment: AT-527 is the orally bioavailable hemi-sulfate salt of AT-511, which is a direct-acting antiviral compound that disrupts activity of the viral RNA polymerase [1]. It was originally developed to combat hepatitis C (HCV) infection, but has been redeployed for SARS-CoV-2. Chemically AT-527 is a phosphoramidate purine nucleotide and it is metabolised to a biologically active guanosine triphosphate analogue in vivo. Preclinical results showing pan-genotype activity against HCV were published in early 2020 [2]. In October 2020 anti-SARS-CoV-2 activity was reported in preprint format [3], which converted to full peer reviewed publication in March 2021 [4].
AT-527 was developed by Atea Pharmaceuticals [5]. In October 2020 it was announced that Roche were purchasing the rights to AT-527 (and gave it the research code RO7496998) outside of the US. In the same press release, it was revealed that AT-527 would be evaluated in Phase 3 trial in early 2021 to determine its efficacy as an early treatment for COVID-19 in non-hospitalised patients. This is a complex molecule with 5 stereo-centres, so there are several CIDs with the same connectivity in PubChem. We chose to represent the molecule from CID 122527275 as it is linked to a FDA UNique Ingredient Identifier (UNII) code. This CID represents the parent molecule. For the hemi-sulfate salt structure see PubChem CID 155926085. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Human safety, tolerability and anti-HCV activity of AT-527 (the prodrug) have been confirmed in a number of HCV-infected patients in a proof-of-concept study [1]. In October 2021, Atea/Roche announced that AT-527 failed to meet the primary outcome of reducing patient viral loads compared to placebo in its mid-stage trial NCT04709835. In light of this disappointing result, the development team indicated that the late-stage MORNINGSKY trial (NCT04889040) protocol would likely be modified to focus on high-risk patients, and potentially exclude those who have received an anti-coronavirus vaccine. Notably this would be in line with the design of trials for two other COVID-19 oral antivirals; molnupiravir which was approved for use in the UK in November 2021, and nirmatrelvir for which significant efficacy in patients at high risk of developing severe disease was also announced in November 2021. The changes will delay the results of MORNINGSKY until the second half of 2022. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03219957 | Study of AT-527 in Healthy and HCV-Infected Subjects | Phase 1 Interventional | Atea Pharmaceuticals, Inc. | 1 | |
| NCT04309734 | Study of AT-777 in Healthy Subjects and AT-777 in Combination With AT-527 in HCV-Infected Subjects | Phase 1/Phase 2 Interventional | Atea Pharmaceuticals, Inc. | ||
| NCT04019717 | Study of AT-527 in Combination With Daclatasvir in Subjects With Hepatitis C Virus (HCV) Infection | Phase 2 Interventional | Atea Pharmaceuticals, Inc. | ||
| NCT04889040 | Study to Evaluate the Effects of RO7496998 (AT-527) in Non-Hospitalized Adult and Adolescent Participants With Mild or Moderate COVID-19 | Phase 3 Interventional | Atea Pharmaceuticals, Inc. | ||
| NCT04396106 | Safety and Efficacy of AT-527 in Subjects With Moderate Coronavirus Disease (COVID-19) | Phase 2 Interventional | Atea Pharmaceuticals, Inc. | In October 2021, Atea/Roche announced that AT-527 failed to meet the primary outcome of reducing patient viral loads compared to placebo in this study. | |
| NCT05059080 | A Six-Month Follow-Up Study of Participants With Coronavirus Disease 2019 (COVID-19) Previously Enrolled in a RO7496998 (AT-527) Study | Observational | Atea Pharmaceuticals, Inc. | ||