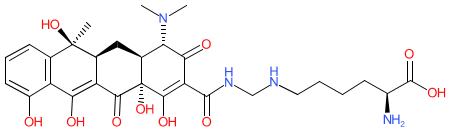
|
Synonyms: N-Lysinomethyltetracycline | tetracycline-L Methylene-Lysine | Tetralysal®
lymecycline is an approved drug (UK (1995))
Compound class:
Synthetic organic
Comment: Lymecycline is a tetracycline-based broad-spectrum antibacterial [3]. It is substantially more soluble than tetracycline, so can be used at lower doses. Lymecycline also has anti-protozoal activity.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Lymecycline is not approved by the FDA or EMA, but holds marketing authorisations from national regulatory agencies, such as the UK, Denmark, Sweden, Norway, France, Ireland, New Zealand and many more. In the UK this drug is used to treat infections caused by tetracycline sensitive organisms, including acne [1-2], ear, nose and throat infections, acute exacerbation of chronic bronchitis, gastro-intestinal infection, urinary tract infection, non-gonococcal urethritis, Rickettsial fever and soft tissue infection. |