|
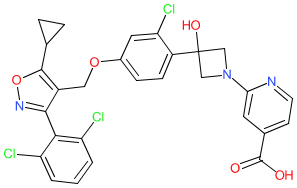
Synonyms: example 13/9 [US10485795B2] | GS-9674 | GS9674
Compound class:
Synthetic organic
Comment: Cilofexor (GS-9674) is a nonsteroidal farnesoid X receptor (FXR) agonist. It was originally identified by Phenex Pharmaceuticals [1] and is being developed by Gilead Pharmaceuticals for hepatic anti-steatotic activity, and potential to treat nonalcoholic fatty liver disease (NAFLD) and/or nonalcoholic steatohepatitis (NASH). Successful treatment of these liver conditions will most likely be achieved using combinations of antilipemic, anti-inflammatory and anti-fibrotic agents.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Cilofexor (GS-9674) has progressed to Phase 3 evaluation in patients with primary sclerosing cholangitis. Phase 2 results in this indication were published in 2019 [3]. Click here to link to ClinicalTrials.gov's full list of GS-9674 studies. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02781584 | Safety, Tolerability, and Efficacy of Selonsertib, Firsocostat, and Cilofexor in Adults With Nonalcoholic Steatohepatitis (NASH) | Phase 2 Interventional | Gilead Sciences | ||
| NCT03890120 | Safety, Tolerability, and Efficacy of Cilofexor in Non-Cirrhotic Adults With Primary Sclerosing Cholangitis | Phase 3 Interventional | Gilead Sciences | ||