|
Synonyms: A35 [WO2012135160A1] | Example 51 [WO2012135160A1] | ME-401 | ME401 | PWT-143 | PWT143
Compound class:
Synthetic organic
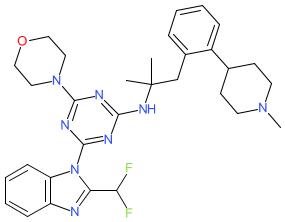
Comment: The chemical structure of example 51 [WO2012135160A1] [1] is identical to the structure that was submitted to the WHO for the INN zandelisib. In the INN submission zandelisib is designated as a PI3 kinase (PI3K) inhibitor and as an antineoplastic. Patent WO2012135160A1 was submitted by the PI3K inhibitor-focussed pharmaceutical company Pathway Therapeutics. Zandelisib has subsequently been declared as MEI Pharma's lead PI3Kδ inhibitor ME-401 [2].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| ME-401 has advanced to clinical evaluation in a number of studies for potential to treat lymphomas. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03768505 | Zandelisib (ME-401) in Subjects With Follicular Lymphoma or Marginal Zone Lymphoma After Failure of Two or More Prior Therapies (TIDAL) | Phase 2 Interventional | MEI Pharma, Inc. | ||
| NCT04533581 | Study of ME-401 in Subjects With Relapsed or Refractory Indolent B-cell Non-Hodgkin's Lymphoma (NHL) | Phase 2 Interventional | Kyowa Kirin Co., Ltd. | ||
| NCT04517435 | ME-401 and R-CHOP in Newly Diagnosed Diffuse Large B-Cell Lymphoma | Phase 1/Phase 2 Interventional | Case Comprehensive Cancer Center | ||
| NCT04745832 | Phase 3 Study of Zandelisib (ME-401) in Combination With Rituximab in Patients With iNHL - (COASTAL) | Phase 3 Interventional | MEI Pharma, Inc. | ||
| NCT02521389 | Assessment of Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Various Formulations and Doses of PWT-143 | Phase 1 Interventional | MEI Pharma, Inc. | 2 | |