|
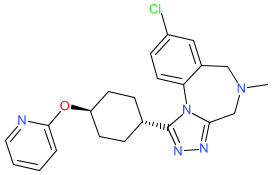
Synonyms: compound 1 [PMID: 31951127] | RG7314 | RO5285119
Compound class:
Synthetic organic
Comment: Balovaptan ((RG7314, RO5285119) is a potent, selective, orally administered and brain-penetrant vasopressin 1A (V1A) receptor antagonist [2]. It was designed by Hoffmann-La Roche as a treatment for autism spectrum disorder, but its clinical development has been terminated.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Encouraging Phase 2 trial results have been published [1]. Phase 3 evaluation of balovaptan's efficacy will be determined in clinical trial NCT03504917, which began in 2018. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03504917 | A Study of Balovaptan in Adults With Autism Spectrum Disorder With a 2-Year Open-Label Extension | Phase 3 Interventional | Hoffmann-La Roche | ||