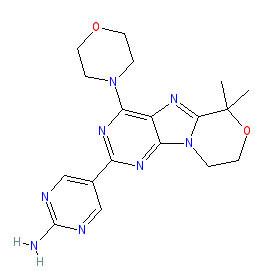
|
Synonyms: compound 16 [PMID: 27096040] | GDC-0084 | GDC0084
Compound class:
Synthetic organic
Comment: Paxalisib (GDC0084) is a dual PI3K/mTOR inhibitor that was developed by Genentech [1]. It was designed to cross the blood-brain-barrier as a potential oncology drug for the treatment of malignant gliomas.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Paxalisib (GDC0084) has progressed to Phase 2 clinical evaluation in patients with newly-diagnosed glioblastoma multiforme (NCT03522298). The drug developer is Kazia Therapeutics Ltd. Kazia received orphan drug designation (ODD) for paxalisib in glioblastoma in February 2018. In August 2020, the FDA extended the ODD to include the treatment the rare and aggressive childhood brain cancer, diffuse intrinsic pontine glioma. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03522298 | Safety, Pharmacokinetics and Efficacy of GDC-0084 in Newly-diagnosed Glioblastoma Multiforme | Phase 2 Interventional | Kazia Therapeutics Limited | ||