|
Compound class:
Synthetic organic
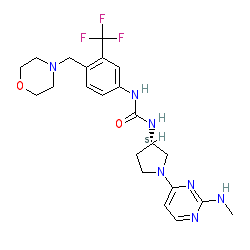
Comment: Compound 20 is a type II inhibitor (binds in a DMG-out mode) of CDK8, with an improved selectivity profile compared to sorafenib [2]. It is one of the compounds claimed in patent WO2015049325 [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| At 1μM, compound 20 inhibits only 2 out of 220 kinases tested by >50% [2]. One of these hits is CDK8/cyclin C, the other is the receptor tyrosine kinase, fms related tyrosine kinase 3 (FLT3). However, it shows only weak anti-proliferative activity in cell assays, which is in concordance with results with other type II CDK8 inhibitors [3]. |
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||