|
Compound class:
Synthetic organic
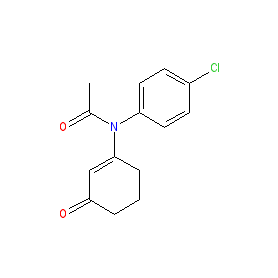
Comment: Compound 9 is an example of a new chemotype (with a cyclic enaminone structural scaffold) for selective COX2 inhibitors (COXIBs) [1]. It is hoped that this new chemotype will be devoid of the side effects related to the sulfonamide/sulfonate group common in existing COXIB drugs, and thus lead the way to overcoming the adverse cardiovascular effects that have led to the withdrawl of some existing COXIBs from use in the clinic (rofecoxib and valdecoxib).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Compound 9 is ~110-fold selective for COX2 compared to COX1 in vitro [1]. |
| Selectivity at enzymes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||