|
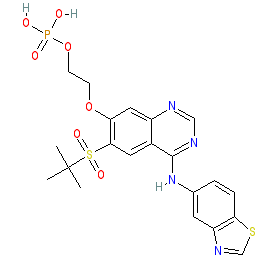
Synonyms: compound 3 [PMID: 31265286] | Example 1 [WO2014043446A1] | GSK2983559
Compound class:
Synthetic organic
Comment: This is an orally active RIPK2 inhibitor phosphate ester prodrug [1-2]. It is rapidly cleaved by alkaline phosphatases on contact with intestinal tissue and in whole blood. The prodrug formulation was designed to limit systemic exposure, and to focus activity at the preferred site of action in gut tissue.
A crystal structure of RIPK2 in complex with inhibitor 3 has been submitted to the PDB with ID 6RN8. |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| In a kinase screening panel VEGFR3 was identified as the only kinase to be inhibited by >90% by inhibitor 3 [2]. Screening against a range of non-kinase targets, revealed that the only receptor to be inhibited at doses <10 μM was the melatonin receptor MT3. MT3 is unlikely to represent a problematic off-target as its expression is largely restricted to the brain, and the active metabolite (RIPK2 inhibitor 5) has low brain penetration potential (in rat tissue distribution model). Inhibitor 3 does not alter hERG or CYP3A4 activity (IC50 >30 μM). As inhibitor 3 is a prodrug, biological activity is most likely ascribed to the active metabolite which is efficiently and rapidly produced from the prodrug at the site of absorption in the gut. |