|
Synonyms: compound 3 [PMID: 30359003] | PQR-620
Compound class:
Synthetic organic
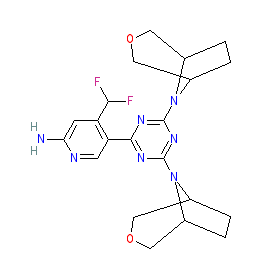
Comment: PQR620 is a potent and selective inhibitor of mTORC1/2 mTOR-containing complexes that was developed by PIQUR Therapeutics [2]. It is brain-penetrating and is being analysed for clinical potential in neurological disorders and cancer. PQR620 was designed as a more effective alternative to the rapalogs sirolimus, everolimus and temsirolimus which show only modest overall response rates in solid tumours [1]. Chemically PQR620 is a rationally designed derivative of bimiralisib (PQR309), in which bimiralisib's trifluoromethyl group is substituted by a difluoromethyl group, and its morpholino groups are substituted with oxo-azabicyclo moieties.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| PQR620 exhibits favourable selectivity for mTOR over PI3K and other protein kinases [2]. It prevents proliferation in a screening panel of 66 cancer cell lines, and is anti-proliferative in a mouse xenograft model of ovarian cancer. Brain exposure is evidenced by PQR620's ability to reduce epileptic seizures in a tuberous sclerosis complex (TSC) mouse model. |
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||