stimulator of interferon response cGAMP interactor 1
 Target has curated data in GtoImmuPdb
Target has curated data in GtoImmuPdb
Target id: 2902
Nomenclature: stimulator of interferon response cGAMP interactor 1
Abbreviated Name: STING
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 4 | 379 | 5q31.2 | STING1 | stimulator of interferon response cGAMP interactor 1 | |
| Mouse | 4 | 378 | 18 | Sting1 | stimulator of interferon response cGAMP interactor 1 | |
| Rat | - | 379 | 18p11 | Sting1 | stimulator of interferon response cGAMP interactor 1 | |
| Gene and Protein Information Comments | ||||||
| For the human and mouse genes alternate splicing is reported to result in multiple transcript variants, and protein isoforms. We provide details for the longest transcripts and proteins here. The Entrez gene links provide full details of other variants identified. | ||||||
Previous and Unofficial Names  |
| Tmem173 | NET23 | STimulator of INterferon Genes | STING | TMEM173 | transmembrane protein 173 |
Database Links  |
|
| Alphafold | Q86WV6 (Hs), Q3TBT3 (Mm) |
| ChEMBL Target | CHEMBL4523377 (Hs), CHEMBL4523311 (Mm) |
| Ensembl Gene | ENSG00000184584 (Hs), ENSMUSG00000024349 (Mm), ENSRNOG00000042137 (Rn) |
| Entrez Gene | 340061 (Hs), 72512 (Mm), 498840 (Rn) |
| Human Protein Atlas | ENSG00000184584 (Hs) |
| KEGG Gene | hsa:340061 (Hs), mmu:72512 (Mm), rno:498840 (Rn) |
| OMIM | 612374 (Hs) |
| Pharos | Q86WV6 (Hs) |
| RefSeq Nucleotide | NM_198282 (Hs), NM_028261 (Mm), NM_001109122 (Rn) |
| RefSeq Protein | NP_938023 (Hs), NP_082537 (Mm), NP_001102592 (Rn) |
| SynPHARM | 83677 (in complex with 2'3'-cGAMP) |
| UniProtKB | Q86WV6 (Hs), Q3TBT3 (Mm) |
| Wikipedia | STING1 (Hs) |
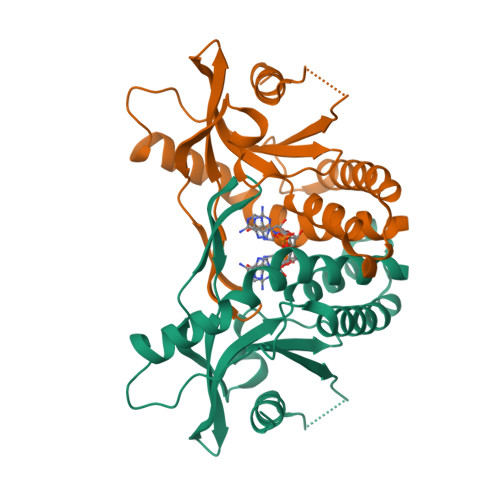
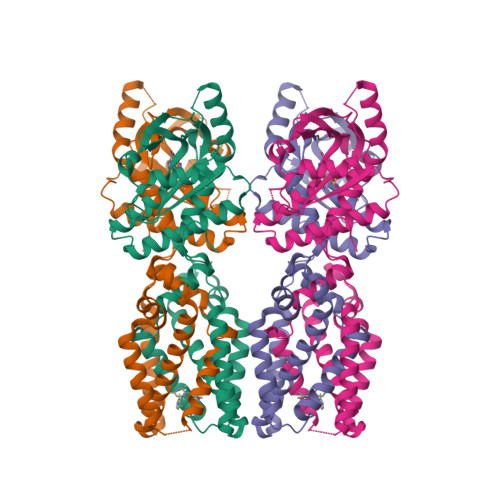
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| STING functions as a major regulator of the innate immune response to viral and bacterial infections [24]. Located in the endoplasmic reticulum, it acts as a pattern recognition receptor, activating type I interferon (IFN) responses upon direct binding to a variety of different cyclic-di-nucleotides (for example of pathogenic origin) [3,5,19-20,33]. Mechanistically, STING mediates DNA-induced TANK-binding kinase 1 (TBK1) activation, which in turn coordinates the phosphorylation-induced activation of IRF3 (an interferon regulatory transcription factor (IRF)) leading to transcriptional regulation of immune response genes including type I IFNs and antiviral IFN-stimulated genes [33]. In cancer, STING is reported to mediate immune recognition of immunogenic tumours, by activating the cyclic-GMP-AMP synthase (cGAS)-STING-interferon regulatory factor 3 (IRF3) pathway in response to the detection of tumor-cell-derived DNA [22,34]. STING sits at the crossroads of infection, inflammation and cancer, hence pharmaceutical industry interest is intense [4]. Pharmacological stimulation of innate type I IFN responses by STING agonists is being investigated as a novel way to combat viral infections [17,29], and for immuno-oncology potential [1,8,12,14-15,26]. Several cyclic dinucleotide-based STING agonists are under scrutiny. Merck's STING agonist ulevostinag (MK-1454) was advanced to clinical evaluaion, however zero activity was detected in NCT03010176 where the compound was evaluated as a monotherapy in patients with solid tumors or lymphomas [16]. Additional trials with ulevostinag in combination with the anti-PD-1 checkpoint inhibitor pembrolizumab were progressed. The mechanistic function of Aduro Biotech's cyclic dinucleotide STING agonist ADU-S100 (PubChem CID 118989191) in its antitumour activity was reported by Sivick et al. in 2018 [31], but as with the Merck competitor, its clinical efficacy was disappointing. Spring Bank Pharmaceuticals' STING agonist (SB 11285) has advanced to Phase 1 evaluation in a variety of solid tumours, either alone or as an adjunct to anti-PD-L1 atezolizumab (NCT04096638). Identification of activating STING mutations as the cause of infantile-onset STING-associated vasculopathy (SAVI) [11,23] has instigated the search for pharmacological inhibitors of STING, as a therapeutic strategy not only for SAVI but also for other DNA-induced type I interferonopathies, including systemic lupus erythematosus [10,21]. cGAS-STING pathway in SARS-CoV-2 infection (COVID-19) The cGAS-STING pathway is reported to drive Type I interferon-mediated immunopathology in COVID-19 [13]. In vivo (in K18-hACE2 transgenic mice) studies revealed that pharmacological inhibition of STING (with the small molecule STING inhibitor H-151) reduced COVID-19-associated severe lung inflammation, and improved disease outcomes. |
| Immuno Process Associations | ||
|
||
|
||
|
||
|
Physiological Functions 
|
||||||||
|
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| General Comments |
| STING is a pattern recognition receptor that is a central signalling component of the intracellular DNA sensing pathway, |
References
1. Ager CR, Reilley MJ, Nicholas C, Bartkowiak T, Jaiswal AR, Curran MA. (2017) Intratumoral STING Activation with T-cell Checkpoint Modulation Generates Systemic Antitumor Immunity. Cancer Immunol Res, 5 (8): 676-684. [PMID:28674082]
2. Allen BK, Kulkarni M, Chamberlain B, Dwight T, Koh C, Samant R, Jernigan F, Rice J, Tan D, Li S et al.. (2022) Design of a systemic small molecule clinical STING agonist using physics-based simulations and artificial intelligence. biorxiv, Preprint. DOI: 10.1101/2022.05.23.493001
3. Barber GN. (2014) STING-dependent cytosolic DNA sensing pathways. Trends Immunol, 35 (2): 88-93. [PMID:24309426]
4. Barber GN. (2015) STING: infection, inflammation and cancer. Nat Rev Immunol, 15 (12): 760-70. [PMID:26603901]
5. Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. (2011) STING is a direct innate immune sensor of cyclic di-GMP. Nature, 478 (7370): 515-8. [PMID:21947006]
6. Chang W, Altman MD, Lesburg CA, Perera SA, Piesvaux JA, Schroeder GK, Wyss DF, Cemerski S, Chen Y, DiNunzio E et al.. (2022) Discovery of MK-1454: A Potent Cyclic Dinucleotide Stimulator of Interferon Genes Agonist for the Treatment of Cancer. J Med Chem, 65 (7): 5675-5689. [PMID:35332774]
7. Charnley AK, Darcy MG, Dodson JW, Dong X, Hughes TV, Kang J, Leister LK, Lian Y, Li Y, Mehlmann JF et al.. (2017) Heterocyclic amides useful as protein modulators. Patent number: WO2017175147A1. Assignee: Glaxosmithkline Intellectual Property Development Limited. Priority date: 07/04/2016. Publication date: 12/10/2017.
8. Corrales L, Gajewski TF. (2015) Molecular Pathways: Targeting the Stimulator of Interferon Genes (STING) in the Immunotherapy of Cancer. Clin Cancer Res, 21 (21): 4774-9. [PMID:26373573]
9. Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ et al.. (2015) Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep, 11 (7): 1018-30. [PMID:25959818]
10. Crow YJ. (2011) Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci, 1238: 91-8. [PMID:22129056]
11. Crow YJ, Casanova JL. (2014) STING-associated vasculopathy with onset in infancy--a new interferonopathy. N Engl J Med, 371 (6): 568-71. [PMID:25029336]
12. Curran E, Chen X, Corrales L, Kline DE, Dubensky Jr TW, Duttagupta P, Kortylewski M, Kline J. (2016) STING Pathway Activation Stimulates Potent Immunity against Acute Myeloid Leukemia. Cell Rep, 15 (11): 2357-66. [PMID:27264175]
13. Domizio JD, Gulen MF, Saidoune F, Thacker VV, Yatim A, Sharma K, Nass T, Guenova E, Schaller M, Conrad C et al.. (2022) The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature, 603 (7899): 145-151. [PMID:35045565]
14. Foote JB, Kok M, Leatherman JM, Armstrong TD, Marcinkowski BC, Ojalvo LS, Kanne DB, Jaffee EM, Dubensky Jr TW, Emens LA. (2017) A STING Agonist Given with OX40 Receptor and PD-L1 Modulators Primes Immunity and Reduces Tumor Growth in Tolerized Mice. Cancer Immunol Res, 5 (6): 468-479. [PMID:28483787]
15. Gadkaree SK, Fu J, Sen R, Korrer MJ, Allen C, Kim YJ. (2017) Induction of tumor regression by intratumoral STING agonists combined with anti-programmed death-L1 blocking antibody in a preclinical squamous cell carcinoma model. Head Neck, 39 (6): 1086-1094. [PMID:28323387]
16. Gogoi H, Mansouri S, Jin L. (2020) The Age of Cyclic Dinucleotide Vaccine Adjuvants. Vaccines (Basel), 8 (3). DOI: 10.3390/vaccines8030453 [PMID:32823563]
17. Guo F, Tang L, Shu S, Sehgal M, Sheraz M, Liu B, Zhao Q, Cheng J, Zhao X, Zhou T et al.. (2017) Activation of Stimulator of Interferon Genes in Hepatocytes Suppresses the Replication of Hepatitis B Virus. Antimicrob Agents Chemother, 61 (10). [PMID:28717041]
18. Haag SM, Gulen MF, Reymond L, Gibelin A, Abrami L, Decout A, Heymann M, van der Goot FG, Turcatti G, Behrendt R et al.. (2018) Targeting STING with covalent small-molecule inhibitors. Nature, 559 (7713): 269-273. [PMID:29973723]
19. Ishikawa H, Barber GN. (2011) The STING pathway and regulation of innate immune signaling in response to DNA pathogens. Cell Mol Life Sci, 68 (7): 1157-65. [PMID:21161320]
20. Ishikawa H, Ma Z, Barber GN. (2009) STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature, 461 (7265): 788-92. [PMID:19776740]
21. Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg MC, Goudin N, Frémond ML, Nitschke P, Molina TJ et al.. (2014) Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest, 124 (12): 5516-20. [PMID:25401470]
22. Larkin B, Ilyukha V, Sorokin M, Buzdin A, Vannier E, Poltorak A. (2017) Cutting Edge: Activation of STING in T Cells Induces Type I IFN Responses and Cell Death. J Immunol, 199 (2): 397-402. [PMID:28615418]
23. Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Montealegre Sanchez GA, Tenbrock K, Wittkowski H, Jones OY, Kuehn HS et al.. (2014) Activated STING in a vascular and pulmonary syndrome. N Engl J Med, 371 (6): 507-18. [PMID:25029335]
24. Liu Y, Lin R, Olagnier D. (2017) RIGulation of STING expression: at the crossroads of viral RNA and DNA sensing pathways. Inflamm Cell Signal, 4 (1): e1491. [PMID:28191486]
25. Lu D, Shang G, Li J, Lu Y, Bai XC, Zhang X. (2022) Activation of STING by targeting a pocket in the transmembrane domain. Nature, 604 (7906): 557-562. [PMID:35388221]
26. Ohkuri T, Ghosh A, Kosaka A, Sarkar SN, Okada H. (2015) Protective role of STING against gliomagenesis: Rational use of STING agonist in anti-glioma immunotherapy. Oncoimmunology, 4 (4): e999523. [PMID:26137417]
27. Pryde DC, Middya S, Banerjee M, Shrivastava R, Basu S, Ghosh R, Yadav DB, Surya A. (2021) The discovery of potent small molecule activators of human STING. Eur J Med Chem, 209: 112869. [PMID:33038794]
28. Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, Tran JL, Moore P, Lehmann S, Eberl HC et al.. (2018) Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature, 564 (7736): 439-443. [PMID:30405246]
29. Sali TM, Pryke KM, Abraham J, Liu A, Archer I, Broeckel R, Staverosky JA, Smith JL, Al-Shammari A, Amsler L et al.. (2015) Characterization of a Novel Human-Specific STING Agonist that Elicits Antiviral Activity Against Emerging Alphaviruses. PLoS Pathog, 11 (12): e1005324. [PMID:26646986]
30. Seo J, Kang JA, Suh DI, Park EB, Lee CR, Choi SA, Kim SY, Kim Y, Park SH, Ye M et al.. (2017) Tofacitinib relieves symptoms of stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy caused by 2 de novo variants in TMEM173. J Allergy Clin Immunol, 139 (4): 1396-1399.e12. [PMID:28041677]
31. Sivick KE, Desbien AL, Glickman LH, Reiner GL, Corrales L, Surh NH, Hudson TE, Vu UT, Francica BJ, Banda T et al.. (2018) Magnitude of Therapeutic STING Activation Determines CD8+ T Cell-Mediated Anti-tumor Immunity. Cell Rep, 25 (11): 3074-3085.e5. [PMID:30540940]
32. Song Z, Wang X, Zhang Y, Gu W, Shen A, Ding C, Li H, Xiao R, Geng M, Xie Z et al.. (2021) Structure-Activity Relationship Study of Amidobenzimidazole Analogues Leading to Potent and Systemically Administrable Stimulator of Interferon Gene (STING) Agonists. J Med Chem, 64 (3): 1649-1669. [PMID:33470814]
33. Tang CH, Zundell JA, Ranatunga S, Lin C, Nefedova Y, Del Valle JR, Hu CC. (2016) Agonist-Mediated Activation of STING Induces Apoptosis in Malignant B Cells. Cancer Res, 76 (8): 2137-52. [PMID:26951929]
34. Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA et al.. (2014) STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity, 41 (5): 830-42. [PMID:25517615]
35. Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. (2013) Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell, 51 (2): 226-35. [PMID:23747010]
How to cite this page
Other pattern recognition receptors: stimulator of interferon response cGAMP interactor 1. Last modified on 05/02/2024. Accessed on 18/04/2024. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=2902.