Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Natural/Endogenous Ligands
- Agonists
- Antagonists
- Allosteric Modulators
- Immunopharmacology Comments
- Immuno Cell Type Associations
- Immuno Process Associations
- Transduction Mechanisms
- Tissue Distribution
- Expression Datasets
- Functional Assays
- Physiological Functions
- Physiological Consequences of Altering Gene Expression
- Phenotypes, Alleles and Disease Models
- Biologically Significant Variants
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 390 | 6q14.1 | HTR1B | 5-hydroxytryptamine receptor 1B | 19,19,25,47,57 |
| Mouse | 7 | 386 | 9 44.61 cM | Htr1b | 5-hydroxytryptamine (serotonin) receptor 1B | 40 |
| Rat | 7 | 386 | 8q31 | Htr1b | 5-hydroxytryptamine receptor 1B | 1,81 |
Previous and Unofficial Names  |
|
| 5-HT1B | 5-HT1DB | HTR1D2 | 5-HT1B serotonin receptor | serotonin receptor 1B | 5-HT1Dβ [57,88] | 5-hydroxytryptamine (serotonin) receptor 1B, G protein-coupled | |
Database Links  |
|
| Specialist databases | |
| GPCRdb | 5ht1b_human (Hs), 5ht1b_mouse (Mm), 5ht1b_rat (Rn) |
| Other databases | |
| Alphafold | P28222 (Hs), P28334 (Mm), P28564 (Rn) |
| ChEMBL Target | CHEMBL1898 (Hs), CHEMBL3708691 (Mm), CHEMBL3459 (Rn) |
| DrugBank Target | P28222 (Hs) |
| Ensembl Gene | ENSG00000135312 (Hs), ENSMUSG00000049511 (Mm), ENSRNOG00000013042 (Rn) |
| Entrez Gene | 3351 (Hs), 15551 (Mm), 25075 (Rn) |
| Human Protein Atlas | ENSG00000135312 (Hs) |
| KEGG Gene | hsa:3351 (Hs), mmu:15551 (Mm), rno:25075 (Rn) |
| OMIM | 182131 (Hs) |
| Pharos | P28222 (Hs) |
| RefSeq Nucleotide | NM_000863 (Hs), NM_010482 (Mm), NM_022225 (Rn) |
| RefSeq Protein | NP_000854 (Hs), NP_034612 (Mm), NP_071561 (Rn) |
| UniProtKB | P28222 (Hs), P28334 (Mm), P28564 (Rn) |
| Wikipedia | HTR1B (Hs) |
Selected 3D Structures  |
|||||||||||||
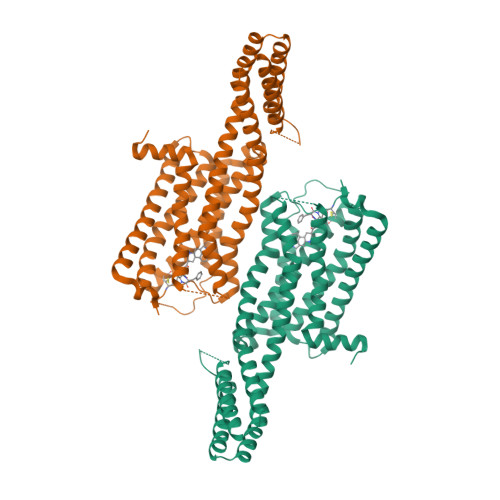
|
|
||||||||||||
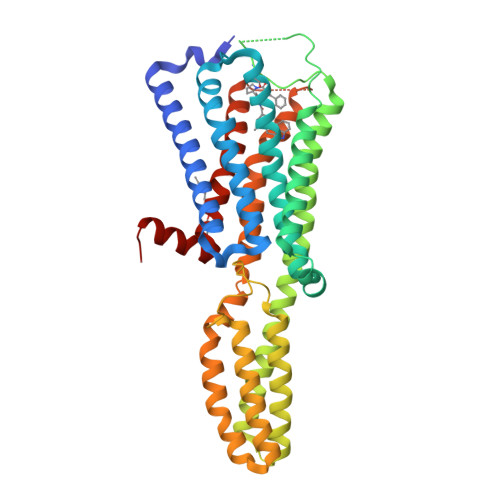
|
|
||||||||||||
Natural/Endogenous Ligands  |
| 5-HT-moduline |
| 5-hydroxytryptamine |
| tryptamine |
| Comments: Endogenous ligand tryptamine is a weak agonist |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Frovatriptan is selective for 1B and 1D 5-HT receptor subtypes compared to the 1A subtype [91]. BRL-15572 can be used to distinguish between 5-HT1D and 5-HT1B receptors, being approximately 60-fold selective for the 1D subtype [61]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| It is possible that radioligand [3H]N-methyl-AZ10419369 may have properties as a partial agonist at the 5-HT1B receptor [37]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulators | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| The expression of 5-HT1B receptors on immune cells indicates that it plays some part in immune/inflammatory responses [83]. |
| Cell Type Associations | ||||||||
|
||||||||
|
||||||||
|
| Immuno Process Associations | ||
|
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gi/Go family | Adenylyl cyclase inhibition |
| References: 34,92 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Biologically Significant Variants 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
References
1. Adham N, Romanienko P, Hartig P, Weinshank RL, Branchek T. (1992) The rat 5-hydroxytryptamine1B receptor is the species homologue of the human 5-hydroxytryptamine1D beta receptor. Mol Pharmacol, 41 (1): 1-7. [PMID:1732716]
2. Ahern GP. (2011) 5-HT and the immune system. Curr Opin Pharmacol, 11 (1): 29-33. [PMID:21393060]
3. Ase AR, Reader TA, Hen R, Riad M, Descarries L. (2000) Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT(1A) or 5-HT(1B) receptor knockout mice. J Neurochem, 75 (6): 2415-26. [PMID:11080193]
4. Bang-Andersen B, Ruhland T, Jørgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mørk A et al.. (2011) Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem, 54 (9): 3206-21. [PMID:21486038]
5. Bartsch T, Knight YE, Goadsby PJ. (2004) Activation of 5-HT(1B/1D) receptor in the periaqueductal gray inhibits nociception. Ann Neurol, 56 (3): 371-81. [PMID:15349864]
6. Belenky MA, Pickard GE. (2001) Subcellular distribution of 5-HT(1B) and 5-HT(7) receptors in the mouse suprachiasmatic nucleus. J Comp Neurol, 432 (3): 371-88. [PMID:11246214]
7. Bonaventure P, Schotte A, Cras P, Leysen JE. (1997) Autoradiographic mapping of 5-HT1B- and 5-HT1D receptors in human brain using [3H]alniditan, a new radioligand. Recept Channels, 5 (3-4): 225-30. [PMID:9606727]
8. Bou J, Domènech T, Puig J, Heredia A, Gras J, Fernández-Forner D, Beleta J, Palacios JM. (2000) Pharmacological characterization of almotriptan: an indolic 5-HT receptor agonist for the treatment of migraine. Eur J Pharmacol, 410 (1): 33-41. [PMID:11134654]
9. Boulenguez P, Segu L, Chauveau J, Morel A, Lanoir J, Delaage M. (1992) Biochemical and pharmacological characterization of serotonin-O-carboxymethylglycyl[125I]iodotyrosinamide, a new radioiodinated probe for 5-HT1B and 5-HT1D binding sites. J Neurochem, 58 (3): 951-9. [PMID:1738002]
10. Bruinvels AT, Palacios JM, Hoyer D. (1993) Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn Schmiedebergs Arch Pharmacol, 347 (6): 569-82. [PMID:8361548]
11. Buhot MC, Naïli S. (1995) Changes in exploratory activity following stimulation of hippocampal 5-HT1A and 5-HT1B receptors in the rat. Hippocampus, 5 (3): 198-208. [PMID:7550615]
12. Chadha A, Sur C, Atack J, Duty S. (2000) The 5HT(1B) receptor agonist, CP-93129, inhibits [(3)H]-GABA release from rat globus pallidus slices and reverses akinesia following intrapallidal injection in the reserpine-treated rat. Br J Pharmacol, 130 (8): 1927-32. [PMID:10952684]
13. Davidson C, Ho M, Price GW, Jones BJ, Stamford JA. (1997) (+)-WAY 100135, a partial agonist, at native and recombinant 5-HT1B/1D receptors. Br J Pharmacol, 121 (4): 737-42. [PMID:9208142]
14. Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N, Abrahamsson PA. (2004) Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate, 59 (3): 328-36. [PMID:15042609]
15. Doménech T, Beleta J, Palacios JM. (1997) Characterization of human serotonin 1D and 1B receptors using [3H]-GR-125743, a novel radiolabelled serotonin 5HT1D/1B receptor antagonist. Naunyn Schmiedebergs Arch Pharmacol, 356 (3): 328-34. [PMID:9303569]
16. El-Khodor BF, Dimmler MH, Amara DA, Hofer M, Hen R, Brunner D. (2004) Juvenile 5HT(1B) receptor knockout mice exhibit reduced pharmacological sensitivity to 5HT(1A) receptor activation. Int J Dev Neurosci, 22 (5-6): 405-13. [PMID:15380839]
17. Gaster LM, Blaney FE, Davies S, Duckworth DM, Ham P, Jenkins S, Jennings AJ, Joiner GF, King FD, Mulholland KR et al.. (1998) The selective 5-HT1B receptor inverse agonist 1'-methyl-5-[[2'-methyl-4'-(5-methyl-1,2, 4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydro- spiro[furo[2,3-f]indole-3,4'-piperidine] (SB-224289) potently blocks terminal 5-HT autoreceptor function both in vitro and in vivo. J Med Chem, 41 (8): 1218-35. [PMID:9548813]
18. Grånäs C, Larhammar D. (1999) Identification of an amino acid residue important for binding of methiothepin and sumatriptan to the human 5-HT(1B) receptor. Eur J Pharmacol, 380 (2-3): 171-81. [PMID:10513577]
19. Hamblin MW, Metcalf MA, McGuffin RW, Karpells S. (1992) Molecular cloning and functional characterization of a human 5-HT1B serotonin receptor: a homologue of the rat 5-HT1B receptor with 5-HT1D-like pharmacological specificity. Biochem Biophys Res Commun, 184 (2): 752-9. [PMID:1315531]
20. Hasegawa Y, Higuchi S, Matsushita S, Miyaoka H. (2002) Association of a polymorphism of the serotonin 1B receptor gene and alcohol dependence with inactive aldehyde dehydrogenase-2. J Neural Transm (Vienna), 109 (4): 513-21. [PMID:11956970]
21. Hawi Z, Dring M, Kirley A, Foley D, Kent L, Craddock N, Asherson P, Curran S, Gould A, Richards S et al.. (2002) Serotonergic system and attention deficit hyperactivity disorder (ADHD): a potential susceptibility locus at the 5-HT(1B) receptor gene in 273 nuclear families from a multi-centre sample. Mol Psychiatry, 7 (7): 718-25. [PMID:12192616]
22. Hou M, Kanje M, Longmore J, Tajti J, Uddman R, Edvinsson L. (2001) 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res, 909 (1-2): 112-20. [PMID:11478927]
23. Hoyer D, Hannon JP, Martin GR. (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav, 71 (4): 533-54. [PMID:11888546]
24. Huang YY, Oquendo MA, Friedman JM, Greenhill LL, Brodsky B, Malone KM, Khait V, Mann JJ. (2003) Substance abuse disorder and major depression are associated with the human 5-HT1B receptor gene (HTR1B) G861C polymorphism. Neuropsychopharmacology, 28 (1): 163-9. [PMID:12496953]
25. Jin H, Oksenberg D, Ashkenazi A, Peroutka SJ, Duncan AM, Rozmahel R, Yang Y, Mengod G, Palacios JM, O'Dowd BF. (1992) Characterization of the human 5-hydroxytryptamine1B receptor. J Biol Chem, 267 (9): 5735-8. [PMID:1348246]
26. John GW, Pauwels PJ, Perez M, Halazy S, Le Grand B, Verscheure Y, Valentin JP, Palmier C, Wurch T, Chopin P et al.. (1999) F 11356, a novel 5-hydroxytryptamine (5-HT) derivative with potent, selective, and unique high intrinsic activity at 5-HT1B/1D receptors in models relevant to migraine. J Pharmacol Exp Ther, 290 (1): 83-95. [PMID:10381763]
27. Johnson SW, Mercuri NB, North RA. (1992) 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci, 12 (5): 2000-6. [PMID:1578282]
28. Jorand-Lebrun C, Pauwels PJ, Palmier C, Moret C, Chopin P, Perez M, Marien M, Halazy S. (1997) 5-HT1B receptor antagonist properties of novel arylpiperazide derivatives of 1-naphthylpiperazine. J Med Chem, 40 (24): 3974-8. [PMID:9397179]
29. Koe BK, Nielsen JA, Macor JE, Heym J. (1992) Biochemical and behavioural studies of the 5-HT1B receptor agonist, CP-94,253. Drug Dev Res, 26: 241-250.
30. Law H, Dukat M, Teitler M, Lee DK, Mazzocco L, Kamboj R, Rampersad V, Prisinzano T, Glennon RA. (1998) Benzylimidazolines as h5-HT1B/1D serotonin receptor ligands: a structure-affinity investigation. J Med Chem, 41 (13): 2243-51. [PMID:9632357]
31. Lee MD, Kennett GA, Dourish CT, Clifton PG. (2002) 5-HT1B receptors modulate components of satiety in the rat: behavioural and pharmacological analyses of the selective serotonin1B agonist CP-94,253. Psychopharmacology (Berl.), 164 (1): 49-60. [PMID:12373419]
32. Lesage AS, Wouters R, Van Gompel P, Heylen L, Vanhoenacker P, Haegeman G, Luyten WH, Leysen JE. (1998) Agonistic properties of alniditan, sumatriptan and dihydroergotamine on human 5-HT1B and 5-HT1D receptors expressed in various mammalian cell lines. Br J Pharmacol, 123 (8): 1655-65. [PMID:9605573]
33. Leysen JE, Gommeren W, Heylen L, Luyten WH, Van de Weyer I, Vanhoenacker P, Haegeman G, Schotte A, Van Gompel P, Wouters R et al.. (1996) Alniditan, a new 5-hydroxytryptamine1D agonist and migraine-abortive agent: ligand-binding properties of human 5-hydroxytryptamine1D alpha, human 5-hydroxytryptamine1D beta, and calf 5-hydroxytryptamine1D receptors investigated with [3H]5-hydroxytryptamine and [3H]alniditan. Mol Pharmacol, 50 (6): 1567-80. [PMID:8967979]
34. Lin SL, Setya S, Johnson-Farley NN, Cowen DS. (2002) Differential coupling of 5-HT(1) receptors to G proteins of the G(i) family. Br J Pharmacol, 136 (7): 1072-8. [PMID:12145108]
35. Lucas JJ, Segu L, Hen R. (1997) 5-Hydroxytryptamine1B receptors modulate the effect of cocaine on c-fos expression: converging evidence using 5-hydroxytryptamine1B knockout mice and the 5-hydroxytryptamine1B/1D antagonist GR127935. Mol Pharmacol, 51 (5): 755-63. [PMID:9145913]
36. Ma QP. (2001) Co-localization of 5-HT(1B/1D/1F) receptors and glutamate in trigeminal ganglia in rats. Neuroreport, 12 (8): 1589-91. [PMID:11409721]
37. Maier DL, Sobotka-Briner C, Ding M, Powell ME, Jiang Q, Hill G, Heys JR, Elmore CS, Pierson ME, Mrzljak L. (2009) [N-methyl-3H3]AZ10419369 binding to the 5-HT1B receptor: in vitro characterization and in vivo receptor occupancy. J Pharmacol Exp Ther, 330 (1): 342-51. [PMID:19401496]
38. Makarenko IG, Meguid MM, Ugrumov MV. (2002) Distribution of serotonin 5-hydroxytriptamine 1B (5-HT(1B)) receptors in the normal rat hypothalamus. Neurosci Lett, 328 (2): 155-9. [PMID:12133578]
39. Manrique C, Héry F, Faudon M, François-Bellan AM. (1999) Indirect evidence for an association of 5-HT(1B) binding sites with retinal and geniculate axon terminals in the rat suprachiasmatic nucleus. Synapse, 33 (4): 314-23. [PMID:10421712]
40. Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. (1992) Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci USA, 89 (7): 3020-4. [PMID:1557407]
41. Maura G, Raiteri M. (1986) Cholinergic terminals in rat hippocampus possess 5-HT1B receptors mediating inhibition of acetylcholine release. Eur J Pharmacol, 129 (3): 333-7. [PMID:3780847]
42. Middlemiss DN, Göthert M, Schlicker E, Scott CM, Selkirk JV, Watson J, Gaster LM, Wyman P, Riley G, Price GW. (1999) SB-236057, a selective 5-HT1B receptor inverse agonist, blocks the 5-HT human terminal autoreceptor. Eur J Pharmacol, 375 (1-3): 359-65. [PMID:10443589]
43. Millan MJ, Gobert A, Newman-Tancredi A, Lejeune F, Cussac D, Rivet JM, Audinot V, Dubuffet T, Lavielle G. (2000) S33084, a novel, potent, selective, and competitive antagonist at dopamine D(3)-receptors: I. Receptorial, electrophysiological and neurochemical profile compared with GR218,231 and L741,626. J Pharmacol Exp Ther, 293 (3): 1048-62. [PMID:10869410]
44. Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. (2002) Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther, 303 (2): 791-804. [PMID:12388666]
45. Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Cogé F, Galizzi JP, Boutin JA, Rivet JM et al.. (2000) Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse, 35 (2): 79-95. [PMID:10611634]
46. Mlinar B, Corradetti R. (2003) Endogenous 5-HT, released by MDMA through serotonin transporter- and secretory vesicle-dependent mechanisms, reduces hippocampal excitatory synaptic transmission by preferential activation of 5-HT1B receptors located on CA1 pyramidal neurons. Eur J Neurosci, 18 (6): 1559-71. [PMID:14511335]
47. Mochizuki D, Yuyama Y, Tsujita R, Komaki H, Sagai H. (1992) Cloning and expression of the human 5-HT1B-type receptor gene. Biochem Biophys Res Commun, 185 (2): 517-23. [PMID:1610347]
48. Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. (2000) Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol Pharmacol, 58 (6): 1271-8. [PMID:11093763]
49. Moser PC, Bergis OE, Jegham S, Lochead A, Duconseille E, Terranova JP, Caille D, Berque-Bestel I, Lezoualc'h F, Fischmeister R et al.. (2002) SL65.0155, a novel 5-hydroxytryptamine(4) receptor partial agonist with potent cognition-enhancing properties. J Pharmacol Exp Ther, 302 (2): 731-41. [PMID:12130738]
50. Napier C, Stewart M, Melrose H, Hopkins B, McHarg A, Wallis R. (1999) Characterisation of the 5-HT receptor binding profile of eletriptan and kinetics of [3H]eletriptan binding at human 5-HT1B and 5-HT1D receptors. Eur J Pharmacol, 368 (2-3): 259-68. [PMID:10193663]
51. Newman-Tancredi A, Audinot V, Moreira C, Verrièle L, Millan MJ. (2000) Inverse agonism and constitutive activity as functional correlates of serotonin h5-HT(1B) receptor/G-protein stoichiometry. Mol Pharmacol, 58 (5): 1042-9. [PMID:11040052]
52. Newman-Tancredi A, Cussac D, Audinot V, Millan MJ. (1999) Actions of roxindole at recombinant human dopamine D2, D3 and D4 and serotonin 5-HT1A, 5-HT1B and 5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol, 359 (6): 447-53. [PMID:10431754]
53. Nilsson T, Longmore J, Shaw D, Olesen IJ, Edvinsson L. (1999) Contractile 5-HT1B receptors in human cerebral arteries: pharmacological characterization and localization with immunocytochemistry. Br J Pharmacol, 128 (6): 1133-40. [PMID:10578124]
54. Nilsson T, Longmore J, Shaw D, Pantev E, Bard JA, Branchek T, Edvinsson L. (1999) Characterisation of 5-HT receptors in human coronary arteries by molecular and pharmacological techniques. Eur J Pharmacol, 372 (1): 49-56. [PMID:10374714]
55. Parker EM, Izzarelli DG, Lewis-Higgins L, Palmer D, Shapiro RA. (1996) Two amino acid differences in the sixth transmembrane domain are partially responsible for the pharmacological differences between the 5-HT1D beta and 5-HT1E 5-hydroxytryptamine receptors. J Neurochem, 67 (5): 2096-103. [PMID:8863519]
56. Pattij T, Broersen LM, van der Linde J, Groenink L, van der Gugten J, Maes RA, Olivier B. (2003) Operant learning and differential-reinforcement-of-low-rate 36-s responding in 5-HT1A and 5-HT1B receptor knockout mice. Behav Brain Res, 141 (2): 137-45. [PMID:12742250]
57. Pauwels PJ, Wurch T, Amoureux MC, Palmier C, Colpaert FC. (1996) Stimulation of cloned human serotonin 5-HT1D beta receptor sites in stably transfected C6 glial cells promotes cell growth. J Neurochem, 66 (1): 65-73. [PMID:8522991]
58. Phebus LA, Johnson KW, Zgombick JM, Gilbert PJ, Van Belle K, Mancuso V, Nelson DL, Calligaro DO, Kiefer Jr AD, Branchek TA et al.. (1997) Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci, 61 (21): 2117-26. [PMID:9395253]
59. Pickard GE, Smith BN, Belenky M, Rea MA, Dudek FE, Sollars PJ. (1999) 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J Neurosci, 19 (10): 4034-45. [PMID:10234032]
60. Pierce PA, Xie GX, Levine JD, Peroutka SJ. (1996) 5-Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neuroscience, 70 (2): 553-9. [PMID:8848158]
61. Price GW, Burton MJ, Collin LJ, Duckworth M, Gaster L, Göthert M, Jones BJ, Roberts C, Watson JM, Middlemiss DN. (1997) SB-216641 and BRL-15572--compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol, 356 (3): 312-20. [PMID:9303567]
62. Quist JF, Barr CL, Schachar R, Roberts W, Malone M, Tannock R, Basile VS, Beitchman J, Kennedy JL. (2003) The serotonin 5-HT1B receptor gene and attention deficit hyperactivity disorder. Mol Psychiatry, 8 (1): 98-102. [PMID:12556913]
63. Roth BL, Ernsberger P, Steinberg SA, Rao S, Rauser L, Savage J, Hufeisen S, Berridge MS, Muzic Jr RF. (2001) The in vitro pharmacology of the beta-adrenergic receptor pet ligand (s)-fluorocarazolol reveals high affinity for cloned beta-adrenergic receptors and moderate affinity for the human 5-HT1A receptor. Psychopharmacology (Berl.), 157 (1): 111-4. [PMID:11512051]
64. Rousselle JC, Plantefol M, Fillion MP, Massot O, Pauwels PJ, Fillion G. (1998) Specific interaction of 5-HT-moduline with human 5-HT1b as well as 5-HT1d receptors expressed in transfected cultured cells. Naunyn Schmiedebergs Arch Pharmacol, 358 (3): 279-86. [PMID:9774213]
65. Russell MG, Matassa VG, Pengilley RR, van Niel MB, Sohal B, Watt AP, Hitzel L, Beer MS, Stanton JA, Broughton HB et al.. (1999) 3-[3-(Piperidin-1-yl)propyl]indoles as highly selective h5-HT(1D) receptor agonists. J Med Chem, 42 (24): 4981-5001. [PMID:10585208]
66. Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Vergé D. (1999) Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience, 88 (3): 899-915. [PMID:10363826]
67. Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. (1994) Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science, 265 (5180): 1875-8. [PMID:8091214]
68. Saxena PR, De Vries P, Wang W, Heiligers JP, Maassen vandenBrink A, Bax WA, Yocca FD. (1997) Effects of avitriptan, a new 5-HT 1B/1D receptor agonist, in experimental models predictive of antimigraine activity and coronary side-effect potential. Naunyn Schmiedebergs Arch Pharmacol, 355 (2): 295-302. [PMID:9050026]
69. Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. (1996) Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl.), 124 (1-2): 57-73. [PMID:8935801]
70. Selkirk JV, Scott C, Ho M, Burton MJ, Watson J, Gaster LM, Collin L, Jones BJ, Middlemiss DN, Price GW. (1998) SB-224289--a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. Br J Pharmacol, 125 (1): 202-8. [PMID:9776361]
71. Shahid M, Walker GB, Zorn SH, Wong EH. (2009) Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol (Oxford), 23 (1): 65-73. [PMID:18308814]
72. Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. (2003) Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology, 28 (8): 1400-11. [PMID:12784105]
73. Shippenberg TS, Hen R, He M. (2000) Region-specific enhancement of basal extracellular and cocaine-evoked dopamine levels following constitutive deletion of the Serotonin(1B) receptor. J Neurochem, 75 (1): 258-65. [PMID:10854269]
74. Singer JH, Bellingham MC, Berger AJ. (1996) Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol, 76 (2): 799-807. [PMID:8871200]
75. Soyka M, Preuss UW, Koller G, Zill P, Bondy B. (2004) Association of 5-HT1B receptor gene and antisocial behavior in alcoholism. J Neural Transm (Vienna), 111 (1): 101-9. [PMID:14714219]
76. Stefulj J, Jernej B, Cicin-Sain L, Rinner I, Schauenstein K. (2000) mRNA expression of serotonin receptors in cells of the immune tissues of the rat. Brain Behav Immun, 14 (3): 219-24. [PMID:10970681]
77. Trillat AC, Malagié I, Scearce K, Pons D, Anmella MC, Jacquot C, Hen R, Gardier AM. (1997) Regulation of serotonin release in the frontal cortex and ventral hippocampus of homozygous mice lacking 5-HT1B receptors: in vivo microdialysis studies. J Neurochem, 69 (5): 2019-25. [PMID:9349547]
78. Varnäs K, Hall H, Bonaventure P, Sedvall G. (2001) Autoradiographic mapping of 5-HT(1B) and 5-HT(1D) receptors in the post mortem human brain using [(3)H]GR 125743. Brain Res, 915 (1): 47-57. [PMID:11578619]
79. Varnäs K, Hurd YL, Hall H. (2005) Regional expression of 5-HT1B receptor mRNA in the human brain. Synapse, 56 (1): 21-8. [PMID:15700286]
80. Varnäs K, Nyberg S, Halldin C, Varrone A, Takano A, Karlsson P, Andersson J, McCarthy D, Smith M, Pierson ME et al.. (2011) Quantitative analysis of [11C]AZ10419369 binding to 5-HT1B receptors in human brain. J Cereb Blood Flow Metab, 31 (1): 113-23. [PMID:20424633]
81. Voigt MM, Laurie DJ, Seeburg PH, Bach A. (1991) Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J, 10 (13): 4017-23. [PMID:1836757]
82. Wang C, Jiang Y, Ma J, Wu H, Wacker D, Katritch V, Han GW, Liu W, Huang XP, Vardy E et al.. (2013) Structural basis for molecular recognition at serotonin receptors. Science, 340 (6132): 610-4. [PMID:23519210]
83. Wang SJ, Sharkey KA, McKay DM. (2018) Modulation of the immune response by helminths: a role for serotonin?. Biosci Rep, 38 (5). DOI: 10.1042/BSR20180027 [PMID:30177522]
84. Ward SE, Harrington FP, Gordon LJ, Hopley SC, Scott CM, Watson JM. (2005) Discovery of the first potent, selective 5-hydroxytryptamine1D receptor antagonist. J Med Chem, 48 (10): 3478-80. [PMID:15887956]
85. Watson J, Brough S, Coldwell MC, Gager T, Ho M, Hunter AJ, Jerman J, Middlemiss DN, Riley GJ, Brown AM. (1998) Functional effects of the muscarinic receptor agonist, xanomeline, at 5-HT1 and 5-HT2 receptors. Br J Pharmacol, 125 (7): 1413-20. [PMID:9884068]
86. Watson J, Roberts C, Scott C, Kendall I, Collin L, Day NC, Harries MH, Soffin E, Davies CH, Randall AD et al.. (2001) SB-272183, a selective 5-HT(1A), 5-HT(1B) and 5-HT(1D) receptor antagonist in native tissue. Br J Pharmacol, 133 (6): 797-806. [PMID:11454652]
87. Watson JM, Burton MJ, Price GW, Jones BJ, Middlemiss DN. (1996) GR127935 acts as a partial agonist at recombinant human 5-HT1D alpha and 5-HT1D beta receptors. Eur J Pharmacol, 314 (3): 365-72. [PMID:8957260]
88. Weinshank RL, Zgombick JM, Macchi MJ, Branchek TA, Hartig PR. (1992) Human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1D alpha and 5-HT1D beta. Proc Natl Acad Sci USA, 89 (8): 3630-4. [PMID:1565658]
89. Wolff M, Benhassine N, Costet P, Hen R, Segu L, Buhot MC. (2003) Delay-dependent working memory impairment in young-adult and aged 5-HT1BKO mice as assessed in a radial-arm water maze. Learn Mem, 10 (5): 401-9. [PMID:14557613]
90. Xie Z, Lee SP, O'Dowd BF, George SR. (1999) Serotonin 5-HT1B and 5-HT1D receptors form homodimers when expressed alone and heterodimers when co-expressed. FEBS Lett, 456 (1): 63-7. [PMID:10452531]
91. Xu YC, Schaus JM, Walker C, Krushinski J, Adham N, Zgombick JM, Liang SX, Kohlman DT, Audia JE. (1999) N-Methyl-5-tert-butyltryptamine: A novel, highly potent 5-HT1D receptor agonist. J Med Chem, 42 (3): 526-31. [PMID:9986723]
92. Zgombick JM, Branchek TA. (1998) Native 5-HT1B receptors expressed in OK cells display dual coupling to elevation of intracellular calcium concentrations and inhibition of adenylate cyclase. Naunyn Schmiedebergs Arch Pharmacol, 358 (5): 503-8. [PMID:9840417]

 Target has curated data in GtoImmuPdb
Target has curated data in GtoImmuPdb














