|
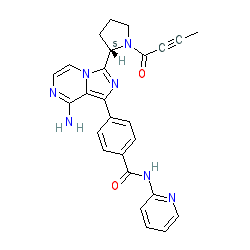
Synonyms: ACP-196 | Calquence® | Example 6 [US20140155385 A1] [2]
acalabrutinib is an approved drug (FDA (2017), EMA (2020))
Compound class:
Synthetic organic
Comment: Acalabrutinib is an orally available second-generation, selective and irreversible inhibitor of Bruton tyrosine kinase (BTK) [6], being investigated for its potential antineoplastic activity (in multiple haematologic malignancies and solid tumours), as well as a potential therapy for rheumatoid arthritis. Acalabrutinib covalently bonds to Cysteine-481 in BTK.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Awan FT, Schuh A, Brown JR, Furman RR, Pagel JM, Hillmen P, Stephens DM, Woyach J, Bibikova E, Charuworn P et al.. (2019)
Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv, 3 (9): 1553-1562. [PMID:31088809] |
|
2. Barf TA, Jans CGJM, Man PADeA, Oubrie AA, Raaijmakers HCA, Rewinkel JBM, Sterrenburg J-G, Wijkmans JCHM. (2014)
4-imidazopyridazin-1-yl-benzamides and 4-imidazotriazin-1-yl-benzamides as btk inhibitors. Patent number: US20140155385 A1. Assignee: Barf TA, Jans CGJM, Man PADeA, Oubrie AA, Raaijmakers HCA, Rewinkel JBM, Sterrenburg J-G, Wijkmans JCHM. Priority date: 19/07/2011. Publication date: 05/06/2014. |
|
3. Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, Chaves J, Wierda WG, Awan FT, Brown JR et al.. (2016)
Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med, 374 (4): 323-32. [PMID:26641137] |
|
4. Sharman JP, Banerji V, Fogliatto LM, Herishanu Y, Munir T, Walewska R, Follows G, Karlsson K, Ghia P, Corbett G et al.. (2019)
ELEVATE TN: Phase 3 Study of Acalabrutinib Combined with Obinutuzumab (O) or Alone Vs O Plus Chlorambucil (Clb) in Patients (Pts) with Treatment-Naive Chronic Lymphocytic Leukemia (CLL). Blood, 134 (Supplement_1): 31. [PMID:31724010] |
|
5. UK Department of Health and Social Care.
COVID-19 treatments could be fast-tracked through new national clinical trial initiative. Accessed on 01/06/2020. Modified on 01/06/2020. gov.uk, https://www.gov.uk/government/news/covid-19-treatments-could-be-fast-tracked-through-new-national-clinical-trial-initiative |
|
6. Wu J, Zhang M, Liu D. (2016)
Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol, 9: 21. [PMID:26957112] |
|
7.
FDA Approves Calquence.
Accessed on 01/11/2017. Modified on 01/11/2017. www.drugs.com, https://www.drugs.com/newdrugs/fda-approves-calquence-acalabrutinib-adults-mantle-cell-lymphoma-4624.html?utm_source=ddc&utm_medium=email&utm_campaign=FDA+Approves+Calquence+%28acalabrutinib%29+for+Adults+with+Mantle+Cell+Lymphoma |