|
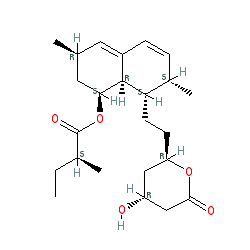
Synonyms: (+)-mevinolin | 6α-methylcompactin | Mevacor®
lovastatin is an approved drug (FDA (1987))
Compound class:
Natural product or derivative
Comment: Lovastatin is a 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA) inhibitor; a statin drug used for the prevention of cardiovascular diseases.
NB: there may be ambiguity in the chiral specification in the reported literature, compared to the structure represented here. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: lovastatin |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E et al.. (1980)
Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA, 77 (7): 3957-61. [PMID:6933445] |
|
2. Bone EA, Cunningham EM, Davidson AH, Galloway WA, Lewis CN, Morrice EM, Reeve M, Todd RS, White IM. (1992)
The design and biological evaluation of a series of 3-hydroxy-3-methylglutaryl coenzyme a (HMG-CoA) reductase inhibitors related to dihydromevinolin. Bioorg Med Chem Lett, 2 (3): 223-228. DOI: 10.1016/S0960-894X(01)81069-6 |
|
3. Choi HW, Shin PG, Lee JH, Choi WS, Kang MJ, Kong WS, Oh MJ, Seo YB, Kim GD. (2018)
Anti-inflammatory effect of lovastatin is mediated via the modulation of NF-κB and inhibition of HDAC1 and the PI3K/Akt/mTOR pathway in RAW264.7 macrophages. Int J Mol Med, 41 (2): 1103-1109. [PMID:29207042] |
|
4. Coppola GM, Damon RE, Yu H, Engstrom RG, Scallen TJ. (1997)
Design and biological evaluation of a series of thiophene-based 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Bioorg Med Chem Lett, 7 (4): 549-554. |
|
5. Jendralla H, Baader E, Bartmann W, Beck G, Bergmann A, Granzer E, von Kerekjarto B, Kesseler K, Krause R, Schubert W. (1990)
Synthesis and biological activity of new HMG-CoA reductase inhibitors. 2. Derivatives of 7-(1H-pyrrol-3-yl)-substituted-3,5-dihydroxyhept-6(E)-enoic (-heptanoic) acids. J Med Chem, 33 (1): 61-70. [PMID:2153213] |
|
6. Jendralla H, Granzer E, von Kerekjarto B, Krause R, Schacht U, Baader E, Bartmann W, Beck G, Bergmann A, Kesseler K. (1991)
Synthesis and biological activity of new HMG-CoA reductase inhibitors. 3. Lactones of 6-phenoxy-3,5-dihydroxyhexanoic acids. J Med Chem, 34 (10): 2962-83. [PMID:1656041] |
|
7. Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. (1998)
The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest, 102 (5): 1016-23. [PMID:9727070] |
|
8. Procopiou PA, Draper CD, Hutson JL, Inglis GG, Ross BC, Watson NS. (1993)
Inhibitors of cholesterol biosynthesis. 2. 3,5-Dihydroxy-7-(N-pyrrolyl)-6-heptenoates, a novel series of HMG-CoA reductase inhibitors. J Med Chem, 36 (23): 3658-62. [PMID:8246234] |
|
9. Sliskovic DR, Blankley CJ, Krause BR, Newton RS, Picard JA, Roark WH, Roth BD, Sekerke C, Shaw MK, Stanfield RL. (1992)
Inhibitors of cholesterol biosynthesis. 6. trans-6-[2-(2-N-heteroaryl-3,5-disubstituted- pyrazol-4-yl)ethyl/ethenyl]tetrahydro-4-hydroxy-2H-pyran-2-ones. J Med Chem, 35 (11): 2095-103. [PMID:1597859] |
|
10. Sliskovic DR, Picard JA, Roark WH, Roth BD, Ferguson E, Krause BR, Newton RS, Sekerke C, Shaw MK. (1991)
Inhibitors of cholesterol biosynthesis. 4. trans-6-[2-(substituted-quinolinyl)ethenyl/ethyl]tetrahydro-4-hydroxy-2 H-pyran-2-ones, a novel series of HMG-CoA reductase inhibitors. J Med Chem, 34 (1): 367-73. [PMID:1992138] |