|
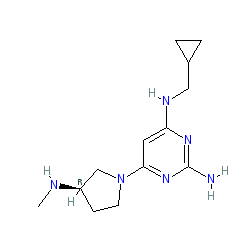
Synonyms: PF 3893787 | PF-03893787 | ZPL-389 | ZPL-3893787 | ZPL389 | ZPL3893787
Compound class:
Synthetic organic
Comment: Adriforant (ZPL-3893787) was a clinical stage immunomodulatory compound being developed by Ziarco Pharma (a Novartis subsidiary). Mechanistically it is a selective histamine H4 receptor antagonist. Novartis announced in their second-quarter financial report of 2020, that they were terminating the adriforant development programme.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| ZPL-3893787 is an invesitgational selective histamine H4 receptor antagonist that is being developed for anti-inflammatory potential in the treatment of dermatological inflammation. |
| Immunopharmacology Disease | |||
| Disease | X-Refs | Comment | References |
| Psoriasis |
Disease Ontology:
DOID:8893 |
Completed Phase 2 trial in psoriasis (see NCT02618616). | |
| Atopic dermatitis |
Disease Ontology:
DOID:3310 OMIM: 603165 |
Completed Phase 2 trial in atopic dermatitis (see NCT02424253). Improvement in inflammatory AD lesions in response to ZPL-3893787 treatment in clinical trial was reported by Werrfel et al. (2018) | 3 |