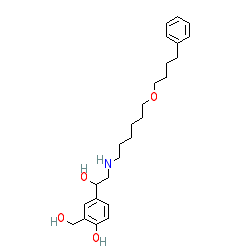
|
Synonyms: GR 33343X | Serevent®
salmeterol is an approved drug (FDA (1994), EMA (2016))
Compound class:
Synthetic organic
Comment: Marketed formulations may contain salmeterol xinafoate (PubChem CID 56801).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: salmeterol |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| As a monotherapy FDA and EMA approvals are for as the use of salmeterol xinafoate. Salmeterol xinafoate is indicated for the treatment of asthma and chronic obstructive pulmonary disease (COPD). Salmeterol is also approved in a fixed-dose inhalation powder formulation with the long-acting β2-adrenergic agonist fluticasone propionate (e.g. AirDuo RespiClickTM or Aerivio SpiromaxTM) for the treatment of severe asthma and COPD. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Causes stimulation of β2 adrenoceptors in the lung leading to relaxation of bronchial smooth muscle, bronchodilation and increased bronchial airflow. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com |