|
Synonyms: Abitrexate® | amethopterin | Nordimet® | Rasuvo®
methotrexate is an approved drug (FDA (1953), EMA (2016))
Compound class:
Synthetic organic
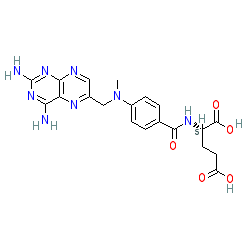
Comment: Methotrexate is a folate (folic acid) analogue that is classified as an antimetabolite and antifolate drug, and a disease-modifying anti-rheumatic drug (DMARD). It is used clinically for its antiproliferative and anti-inflammatory effects. The primary action of methotrexate is inhibition of the enzyme dihydrofolate reductase (DHFR). DHFR is essential for the production of precursors that are required for de novo purine synthesis.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: methotrexate |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Anti-tumour agent used in the treatment of acute lymphocytic leukemia (ALL), meningeal leukemia, non-Hogkin's lymphoma, breast, lung and head and neck cancers. Also indicated in the treatment of gestational choriocarcinoma, chorioadenoma destruens and hydatidiform mole, and in the treatment of autoimmune conditions including severe psoriasis and rheumatoid arthritis. The first oral methotraxate solution (Xatmep®) was FDA approved in April 2017 for the treatment of ALL and polyarticular juvenile idiopathic arthritis (pJIA) in pediatric patients. A 2017 a paper reported that a combination of methotrexate with leflunomide relieves the immune defects and ameliorates symptoms of rheumatoid arthritis [9]. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |