Acetylcholine receptors (muscarinic): Introduction
General
Muscarinic receptors responding to the natural ligand acetylcholine have a widespread tissue distribution and are involved in the control of numerous central and peripheral physiological responses, as well as being a major drug target in human disease. It has been known for some time that this family of G-protein coupled receptors consists of five members designated M1-M5 [2-4,9,28]. The gene family as a whole shows 26.3% overall amino acid identity, with the variation between the receptor subtypes being seen largely within the intracellular loops. The third intracellular loop is particularly variable, showing only 2.7% identity between receptor, compared with an average of 66% identity found in the conserved transmembrane domains. Classically these receptors are sub-divided into two broad groups based on their primary coupling efficiency to G-proteins. Hence, M2 and M4-muscarinic receptors are able to couple to the pertusiss-toxin sensitive Gi/o-proteins, and M1, M3 and M5-muscarinic receptors couple to Gq/11-proteins [9,34]. It is, however, clear that the muscarinic receptor family can couple to a wide range of diverse signalling pathways, some of which are mediated by G-proteins and others that are G-protein-independent [27,31-32].
Muscarinic receptor pharmacology
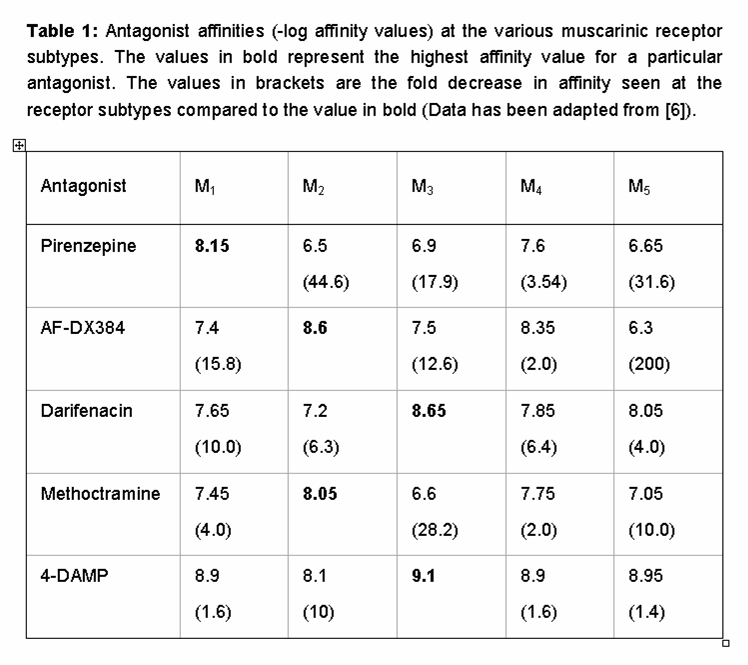
The orthosteric binding pocket of the muscarinic receptor family is highly conserved, making the development of subtype-specific agonists and antagonists very difficult. Table 1 shows the binding affinities of some of the most widely used muscarinic receptor ligands and emphasizes the modest extent of subtype specificity of these agents. In the case of pirenzepine, for example, this antagonist has a 44-fold selectivity for the M1-muscarinic receptor over the M2 , but only a 17.9 and 3.5 fold selectivity for the M3 and M4-muscarinic receptor subtypes respectively [9]. Similarly, darifenacin, which is widely considered as M3 selective and is approved for clinical use in the treatment of over-active bladder has ≤10-fold selectivity over M1/M4/M5 receptors [17].
Attempts to generate novel muscarinic ligands have more recently centred on the development of allosteric regulators that act at non-conserved sites and therefore offer the prospect of subtype selectivity [1,24]. By targeting allosteric sites of muscarinic receptors, contained on the extracellular loops and extracellular regions of the transmembrane helices [24], allosteric modulators are able to regulate the binding affinity of ligands at the orthosteric site. For example, in the presence of the allosteric modulator thiochrome the affinity of acetylcholine at the M4-muscarinic receptor is increased, but the affinity of acetylcholine for the other muscarinic receptor subtypes is unaffected [21]. In addition, subtype-selective agonists, which bind to and activate the receptor via binding domains distinct from the orthosteric binding site have also been reported. These include the highly M1-selective allosteric agonists AC-42 [20,29] and TBPB [18]. These novel agonists have considerable therapeutic potential, but how their signalling properties compare to orthosterically acting agents are only beginning to be explored [30].
Thus, the potential therapeutic benefit of allosteric modulators/agonists is that they show subtype selectivity in a manner that has not be achievable with orthosteric ligands. In addition, the allosteric modulators that act by regulating the affinity of the natural ligand will have the potential to only be active when and where acetylcholine is present and therefore reduce potential side effects [24].
Muscarinic receptor physiological role
The role of muscarinic receptors in the contraction of smooth muscle, particularly of airway, ileum, iris and bladder, are considered a classical muscarinic response mediated primarily by M3-muscarinic receptors expressed on the smooth muscle cells [8,11]. Co-expressed with the M3-muscarinic receptors in smooth muscle is an often larger population of M2-muscarinic receptors [11] which appear to play a much smaller role in the smooth muscle contractile response [35]. In contrast, M2-muscarinic receptors expressed in the heart have a profound role in the control of cardiac myocyte contraction [5,8,35]. Here the release of acetylcholine from vagal parasympathetic neurones reduces heart beating frequency almost exclusively by acting at M2-muscarinic receptors [5,9,33]. Exocrine secretion, particularly of saliva [14,22], and as shown more recently, insulin [12-13], is primarily mediated by M3-muscarinic receptors with a smaller role played by M1-receptors particularly in salvation [14].
The generation of transgenic muscarinic receptor knockout mice, where M1-M5 genes have been ablated, has revealed numerous novel muscarinic receptor functions [23,35-36]. Muscarinic receptor knockout mice are viable and fertile with no major physiological defects, allowing the study of the physiological role of these receptors in adult mice in vivo. Particularly intriguing have been the numerous behavioural and neurological phenotypes observed, revealing the important neuromodulatory role played by this receptor family. M1-muscarinic receptor knockout mice demonstrate a pronounced increase in locomotor activity [26], which has been suggested to impact on memory and learning and may provide a model for learning deficits in conditions such as attention deficit hyperactivity disorder [35-36]. Further locomotor phenotypes are observed in M4-muscarinic receptor knockout animals, where this receptor subtype is thought to mediate an inhibitory affect on striatal dopamine-mediated locomotor activity [6,16,19,36]. M2-muscarinic receptors expressed in the thermo-regulatory centres of the hypothalamus are likely to be involved in the regulation of body temperature [15], whereas M3-muscarinic receptors have been reported to modulate appetite by the regulation of the melanin-concentrating hormone (MCH) neurones in the hypothalamic feeding centre [37].
Muscarinic receptors as drug targets
In line with a greater understanding of the neuromodulatory role of muscarinic receptors has come a greater focus on the possibility that this receptor family may be effective therapeutic targets in a number of neurological and psychiatric diseases [36]. Historically this focus has centred on Alzheimer's disease, which is associated with a loss of cholinergic innervation in the cerebral cortex and hippocampus, and is currently treated by enhancing cholinergic transmission via pharmacological inhibition of cholinesterase activity[25]. Whereas for many years this treatment was thought to have its beneficial affects by stimulation of the M1-muscarinic receptor subtype [7], work on the M1-receptor knockout mice has suggested that this receptor subtype may not play such an important role in cognition as previously thought [36]. Thus, novel anti-Alzheimer drugs that target other muscarinic receptor subtypes, such as the M2-, M4- and M5-muscarinic receptors, are now under more intense consideration. Interestingly, muscarinic receptor modulation of dopaminergic transmission has provided the impetus for the development of muscarinic receptor ligands in the treatment of schizophrenia and Parkinson's disease [36]. In both cases the focus has been on the M1 and M4-muscarinic receptors, where it has been indicated that agonists to these receptors might be beneficial in schizophrenia and antagonists are likely to be of benefit in Parkinson's disease.
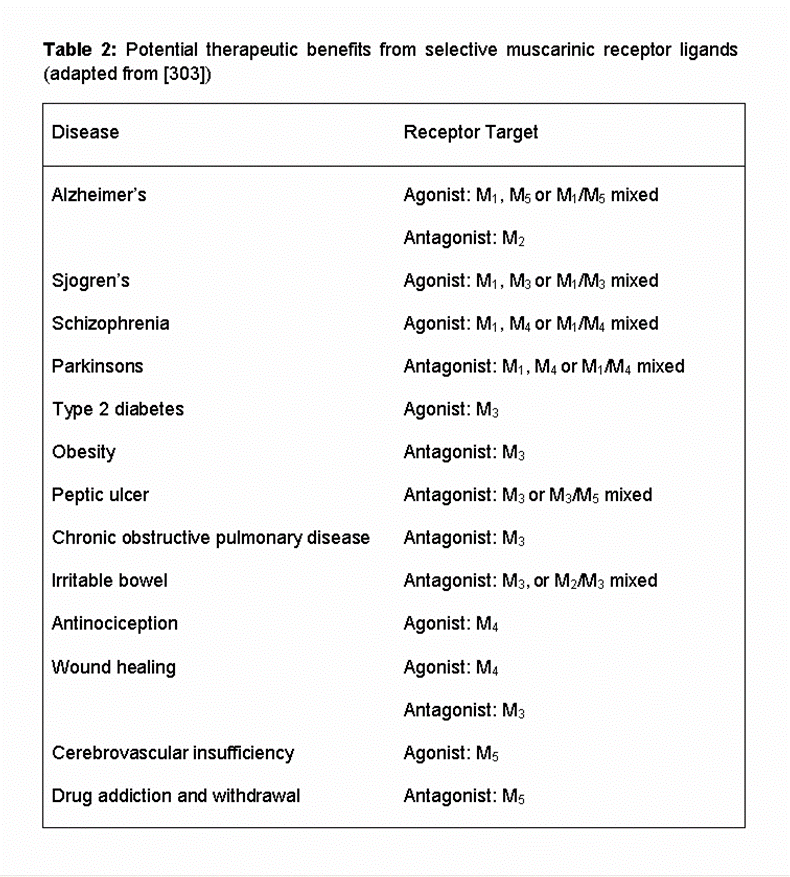
In addition to the therapeutic potential of targeting specific muscarinic receptors in CNS disorders there is still considerable therapeutic potential in the development of muscarinic ligands to the more classical muscarinic-centred disease targets. For example, in the case of chronic obstructive pulmonary disease (COPD) and asthma, that are currently treated with the non-selective muscarinic antagonists ipratropium and tiotropium [10], there might be significant clinical benefit derived from the development of selective M2 and M3-muscarinic receptor ligands. Similarly, in overactive bladder, improved selectivity of muscarinic receptor ligands would help to reduce unwanted side effects [10]. Table 2 (adapted from ref [36]) provides a list of the potential therapeutic applications of compounds that specifically target muscarinic receptor subtypes.
References
1. Birdsall NJ, Lazareno S. (2005) Allosterism at muscarinic receptors: ligands and mechanisms. Mini Rev Med Chem, 5 (6): 523-43. [PMID:15974931]
2. Bonner TI. (1989) The molecular basis of muscarinic receptor diversity. Trends Neurosci, 12 (4): 148-51. [PMID:2470172]
3. Bonner TI, Buckley NJ, Young AC, Brann MR. (1987) Identification of a family of muscarinic acetylcholine receptor genes. Science, 237 (4814): 527-32. [PMID:3037705]
4. Bonner TI, Young AC, Brann MR, Buckley NJ. (1988) Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron, 1 (5): 403-10. [PMID:3272174]
5. Brodde OE, Michel MC. (1999) Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev, 51 (4): 651-90. [PMID:10581327]
6. Bymaster FP, Felder C, Ahmed S, McKinzie D. (2002) Muscarinic receptors as a target for drugs treating schizophrenia. Curr Drug Targets CNS Neurol Disord, 1 (2): 163-81. [PMID:12769625]
7. Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. (2006) M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron, 49 (5): 671-82. [PMID:16504943]
8. Caulfield MP. (1993) Muscarinic receptors--characterization, coupling and function. Pharmacol Ther, 58 (3): 319-79. [PMID:7504306]
9. Caulfield MP, Birdsall NJ. (1998) International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev, 50 (2): 279-90. [PMID:9647869]
10. Eglen RM. (2005) Muscarinic receptor subtype pharmacology and physiology. Prog Med Chem, 43: 105-36. [PMID:15850824]
11. Eglen RM, Hegde SS, Watson N. (1996) Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev, 48 (4): 531-65. [PMID:8981565]
12. Gautam D, Han SJ, Duttaroy A, Mears D, Hamdan FF, Li JH, Cui Y, Jeon J, Wess J. (2007) Role of the M3 muscarinic acetylcholine receptor in beta-cell function and glucose homeostasis. Diabetes Obes Metab, 9 Suppl 2: 158-69. [PMID:17919190]
13. Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C et al.. (2006) A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab, 3 (6): 449-61. [PMID:16753580]
14. Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. (2004) Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol, 66 (2): 260-7. [PMID:15266016]
15. Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. (1999) Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA, 96 (4): 1692-7. [PMID:9990086]
16. Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. (1999) Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA, 96 (18): 10483-8. [PMID:10468635]
17. Hegde SS. (2006) Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol, 147 Suppl 2: S80-7. [PMID:16465186]
18. Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR et al.. (2008) Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci, 28 (41): 10422-33. [PMID:18842902]
19. Karasawa H, Taketo MM, Matsui M. (2003) Loss of anti-cataleptic effect of scopolamine in mice lacking muscarinic acetylcholine receptor subtype 4. Eur J Pharmacol, 468 (1): 15-9. [PMID:12729838]
20. Langmead CJ, Fry VA, Forbes IT, Branch CL, Christopoulos A, Wood MD, Herdon HJ. (2006) Probing the molecular mechanism of interaction between 4-n-butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl]-piperidine (AC-42) and the muscarinic M(1) receptor: direct pharmacological evidence that AC-42 is an allosteric agonist. Mol Pharmacol, 69 (1): 236-46. [PMID:16207821]
21. Lazareno S, Dolezal V, Popham A, Birdsall NJ. (2004) Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol Pharmacol, 65 (1): 257-66. [PMID:14722259]
22. Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. (2000) Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA, 97 (17): 9579-84. [PMID:10944224]
23. Matsui M, Yamada S, Oki T, Manabe T, Taketo MM, Ehlert FJ. (2004) Functional analysis of muscarinic acetylcholine receptors using knockout mice. Life Sci, 75 (25): 2971-81. [PMID:15474550]
24. May LT, Leach K, Sexton PM, Christopoulos A. (2007) Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol, 47: 1-51. [PMID:17009927]
25. Messer WS. (2002) Cholinergic agonists and the treatment of Alzheimer's disease. Curr Top Med Chem, 2: 353-358. [PMID:11966459]
26. Miyakawa T, Yamada M, Duttaroy A, Wess J. (2001) Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci, 21 (14): 5239-50. [PMID:11438599]
27. Nathanson NM. (2000) A multiplicity of muscarinic mechanisms: enough signaling pathways to take your breath away. Proc Natl Acad Sci USA, 97 (12): 6245-7. [PMID:10841527]
28. Peralta EG, Ashkenazi A, Winslow JW, Smith DH, Ramachandran J, Capon DJ. (1987) Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J, 6 (13): 3923-9. [PMID:3443095]
29. Spalding TA, Trotter C, Skjaerbaek N, Messier TL, Currier EA, Burstein ES, Li D, Hacksell U, Brann MR. (2002) Discovery of an ectopic activation site on the M(1) muscarinic receptor. Mol Pharmacol, 61 (6): 1297-302. [PMID:12021390]
30. Thomas RL, Mistry R, Langmead CJ, Wood MD, Challiss RA. (2008) G protein coupling and signaling pathway activation by m1 muscarinic acetylcholine receptor orthosteric and allosteric agonists. J Pharmacol Exp Ther, 327 (2): 365-74. [PMID:18664591]
31. Tobin AB, Budd DC. (2003) The anti-apoptotic response of the Gq/11-coupled muscarinic receptor family. Biochem Soc Trans, 31 (Pt 6): 1182-5. [PMID:14641022]
32. van Koppen CJ, Kaiser B. (2003) Regulation of muscarinic acetylcholine receptor signaling. Pharmacol Ther, 98 (2): 197-220. [PMID:12725869]
33. Wang Z, Shi H, Wang H. (2004) Functional M3 muscarinic acetylcholine receptors in mammalian hearts. Br J Pharmacol, 142 (3): 395-408. [PMID:15148264]
34. Wess J. (1996) Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol, 10 (1): 69-99. [PMID:8853955]
35. Wess J. (2004) Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol, 44: 423-50. [PMID:14744253]
36. Wess J, Eglen RM, Gautam D. (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov, 6 (9): 721-33. [PMID:17762886]
37. Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN et al.. (2001) Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature, 410 (6825): 207-12. [PMID:11242080]







